Tin Kona Island on:
[Wikipedia]
[Google]
[Amazon]
Tin is a chemical element with the
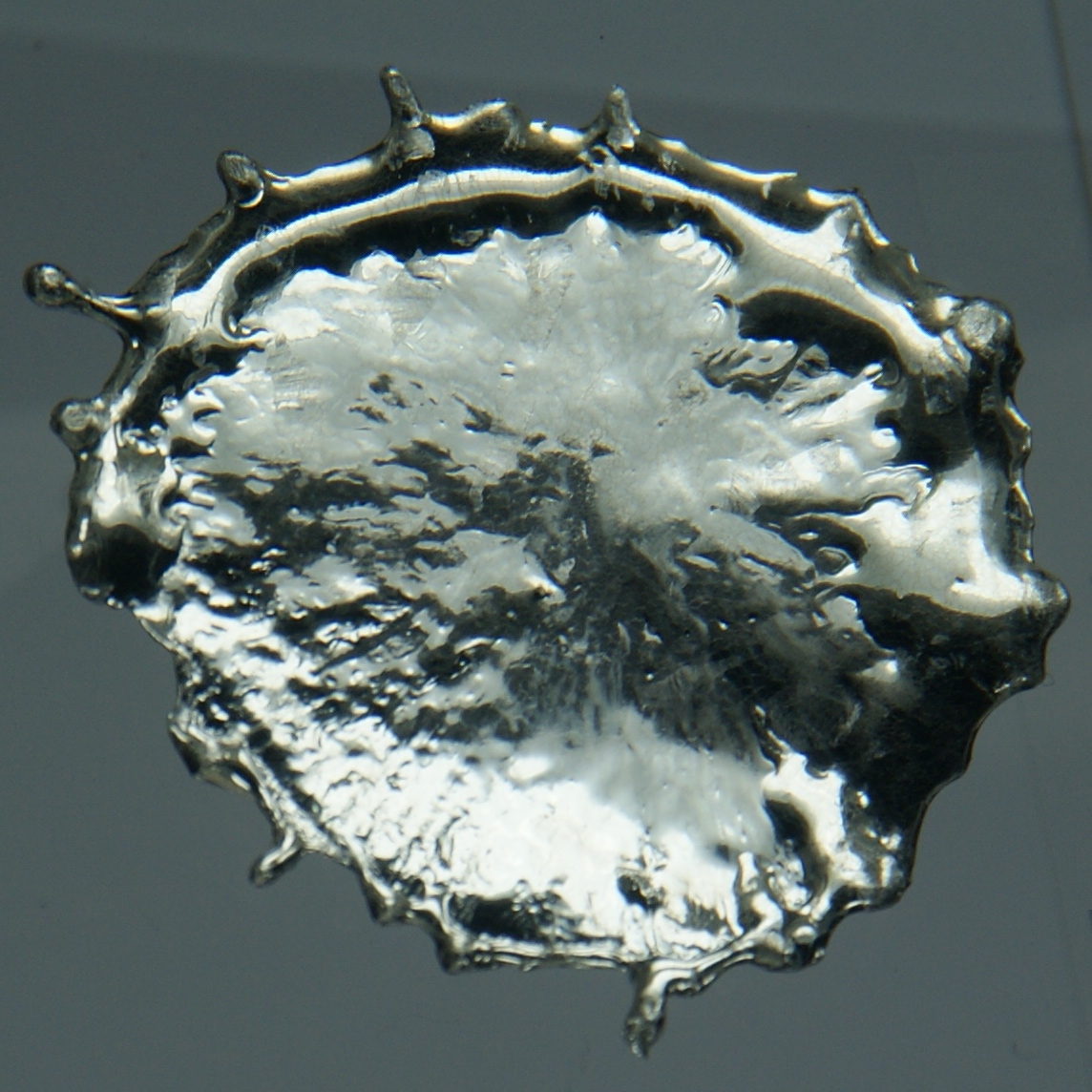 Tin is a soft, malleable, ductile and highly crystalline silvery-white metal. When a bar of tin is bent a crackling sound known as the " tin cry" can be heard from the twinning of the crystals. Tin melts at about the lowest in group 14. The melting point is further lowered to for 11 nm particles.
β-tin, the metallic form or white tin, has BCT structure and is stable at and above room temperature and is malleable. α-tin, the nonmetallic form or gray tin, is stable below and is brittle. α-tin has a diamond cubic crystal structure, similar to diamond, silicon or
Tin is a soft, malleable, ductile and highly crystalline silvery-white metal. When a bar of tin is bent a crackling sound known as the " tin cry" can be heard from the twinning of the crystals. Tin melts at about the lowest in group 14. The melting point is further lowered to for 11 nm particles.
β-tin, the metallic form or white tin, has BCT structure and is stable at and above room temperature and is malleable. α-tin, the nonmetallic form or gray tin, is stable below and is brittle. α-tin has a diamond cubic crystal structure, similar to diamond, silicon or
 Tin extraction and use can be dated to the beginnings of the Bronze Age around 3000 BC, when it was observed that copper objects formed of polymetallic
Tin extraction and use can be dated to the beginnings of the Bronze Age around 3000 BC, when it was observed that copper objects formed of polymetallic

 Tin is generated via the long ''s''-process in low-to-medium mass stars (with masses of 0.6 to 10 times that of the Sun), and finally by beta decay of the heavy isotopes of indium.
Tin is the 49th most abundant element in
Tin is generated via the long ''s''-process in low-to-medium mass stars (with masses of 0.6 to 10 times that of the Sun), and finally by beta decay of the heavy isotopes of indium.
Tin is the 49th most abundant element in
 Tin is unique among mineral commodities because of the complex agreements between producer countries and consumer countries dating back to 1921. Earlier agreements tended to be somewhat informal and led to the "First International Tin Agreement" in 1956, the first of a series that effectively collapsed in 1985. Through these agreements, the International Tin Council (ITC) had a considerable effect on tin prices. ITC supported the price of tin during periods of low prices by buying tin for its buffer stockpile and was able to restrain the price during periods of high prices by selling from the stockpile. This was an anti-free-market approach, designed to assure a sufficient flow of tin to consumer countries and a profit for producer countries. However, the buffer stockpile was not sufficiently large, and during most of those 29 years tin prices rose, sometimes sharply, especially from 1973 through 1980 when rampant inflation plagued many world economies.
During the late 1970s and early 1980s, the U.S. reduced its strategic tin stockpile, partly to take advantage of historically high tin prices. The 1981–82 recession damaged the tin industry. Tin consumption declined dramatically. ITC was able to avoid truly steep declines through accelerated buying for its buffer stockpile; this activity required extensive borrowing. ITC continued to borrow until late 1985 when it reached its credit limit. Immediately, a major "tin crisis" ensued — tin was delisted from trading on the London Metal Exchange for about three years. ITC dissolved soon afterward, and the price of tin, now in a free-market environment, fell to $4 per pound and remained around that level through the 1990s. The price increased again by 2010 with a rebound in consumption following the 2007–2008 economic crisis, accompanying restocking and continued growth in consumption.
Tin is unique among mineral commodities because of the complex agreements between producer countries and consumer countries dating back to 1921. Earlier agreements tended to be somewhat informal and led to the "First International Tin Agreement" in 1956, the first of a series that effectively collapsed in 1985. Through these agreements, the International Tin Council (ITC) had a considerable effect on tin prices. ITC supported the price of tin during periods of low prices by buying tin for its buffer stockpile and was able to restrain the price during periods of high prices by selling from the stockpile. This was an anti-free-market approach, designed to assure a sufficient flow of tin to consumer countries and a profit for producer countries. However, the buffer stockpile was not sufficiently large, and during most of those 29 years tin prices rose, sometimes sharply, especially from 1973 through 1980 when rampant inflation plagued many world economies.
During the late 1970s and early 1980s, the U.S. reduced its strategic tin stockpile, partly to take advantage of historically high tin prices. The 1981–82 recession damaged the tin industry. Tin consumption declined dramatically. ITC was able to avoid truly steep declines through accelerated buying for its buffer stockpile; this activity required extensive borrowing. ITC continued to borrow until late 1985 when it reached its credit limit. Immediately, a major "tin crisis" ensued — tin was delisted from trading on the London Metal Exchange for about three years. ITC dissolved soon afterward, and the price of tin, now in a free-market environment, fell to $4 per pound and remained around that level through the 1990s. The price increased again by 2010 with a rebound in consumption following the 2007–2008 economic crisis, accompanying restocking and continued growth in consumption.
 London Metal Exchange (LME) is tin's principal trading site. Other tin contract markets are Kuala Lumpur Tin Market (KLTM) and Indonesia Tin Exchange (INATIN).
Due to factors involved in the
London Metal Exchange (LME) is tin's principal trading site. Other tin contract markets are Kuala Lumpur Tin Market (KLTM) and Indonesia Tin Exchange (INATIN).
Due to factors involved in the
 Tin has long been used in alloys with lead as solder, in amounts of 5 to 70% w/w. Tin with lead forms a eutectic mixture at the weight proportion of 61.9% tin and 38.1% lead (the atomic proportion: 73.9% tin and 26.1% lead), with melting temperature of 183 °C (361.4 °F). Such solders are primarily used for joining pipes or electric circuits. Since the European Union Waste Electrical and Electronic Equipment Directive (WEEE Directive) and Restriction of Hazardous Substances Directive came into effect on 1 July 2006, the lead content in such alloys has decreased. While lead exposure is associated with serious health problems, lead-free solder is not without its challenges, including a higher melting point, and the formation of tin whiskers that cause electrical problems. Tin pest can occur in lead-free solders, leading to loss of the soldered joint. Replacement alloys are being found, but the problems of joint integrity remain.
Tin has long been used in alloys with lead as solder, in amounts of 5 to 70% w/w. Tin with lead forms a eutectic mixture at the weight proportion of 61.9% tin and 38.1% lead (the atomic proportion: 73.9% tin and 26.1% lead), with melting temperature of 183 °C (361.4 °F). Such solders are primarily used for joining pipes or electric circuits. Since the European Union Waste Electrical and Electronic Equipment Directive (WEEE Directive) and Restriction of Hazardous Substances Directive came into effect on 1 July 2006, the lead content in such alloys has decreased. While lead exposure is associated with serious health problems, lead-free solder is not without its challenges, including a higher melting point, and the formation of tin whiskers that cause electrical problems. Tin pest can occur in lead-free solders, leading to loss of the soldered joint. Replacement alloys are being found, but the problems of joint integrity remain.
 Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. Tin-plated (or tinning) steel containers is widely used for
Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. Tin-plated (or tinning) steel containers is widely used for

 Tin in combination with other elements forms a wide variety of useful alloys. Tin is most commonly alloyed with copper. Pewter is 85–99% tin; bearing metal has a high percentage of tin as well.
Tin in combination with other elements forms a wide variety of useful alloys. Tin is most commonly alloyed with copper. Pewter is 85–99% tin; bearing metal has a high percentage of tin as well.
 Punched tin-plated steel, also called pierced tin, is an artisan technique originating in central Europe for creating functional and decorative housewares. Decorative piercing designs exist in a wide variety, based on local tradition and the artisan. Punched tin lanterns are the most common application of this artisan technique. The light of a candle shining through the pierced design creates a decorative light pattern in the room where it sits. Lanterns and other punched tin articles were created in the New World from the earliest European settlement. A well-known example is the Revere lantern, named after Paul Revere.
Before the modern era, in some areas of the Alps, a goat or sheep's horn would be sharpened and a tin panel would be punched out using the alphabet and numbers from one to nine. This learning tool was known appropriately as "the horn". Modern reproductions are decorated with such motifs as hearts and tulips.
In America, pie safes and food safes were in use in the days before refrigeration. These were wooden cupboards of various styles and sizes – either floor standing or hanging cupboards meant to discourage vermin and insects and to keep dust from perishable foodstuffs. These cabinets had tinplate inserts in the doors and sometimes in the sides, punched out by the homeowner, cabinetmaker, or a tinsmith in varying designs to allow for air circulation while excluding flies. Modern reproductions of these articles remain popular in North America.
Window glass is most often made by floating molten glass on molten tin (
Punched tin-plated steel, also called pierced tin, is an artisan technique originating in central Europe for creating functional and decorative housewares. Decorative piercing designs exist in a wide variety, based on local tradition and the artisan. Punched tin lanterns are the most common application of this artisan technique. The light of a candle shining through the pierced design creates a decorative light pattern in the room where it sits. Lanterns and other punched tin articles were created in the New World from the earliest European settlement. A well-known example is the Revere lantern, named after Paul Revere.
Before the modern era, in some areas of the Alps, a goat or sheep's horn would be sharpened and a tin panel would be punched out using the alphabet and numbers from one to nine. This learning tool was known appropriately as "the horn". Modern reproductions are decorated with such motifs as hearts and tulips.
In America, pie safes and food safes were in use in the days before refrigeration. These were wooden cupboards of various styles and sizes – either floor standing or hanging cupboards meant to discourage vermin and insects and to keep dust from perishable foodstuffs. These cabinets had tinplate inserts in the doors and sometimes in the sides, punched out by the homeowner, cabinetmaker, or a tinsmith in varying designs to allow for air circulation while excluding flies. Modern reproductions of these articles remain popular in North America.
Window glass is most often made by floating molten glass on molten tin (
Tin
at ''The Periodic Table of Videos'' (University of Nottingham)
Theodore Gray's Wooden Periodic Table Table
Tin samples and castings
Tin (USD cents per kg)
{{Authority control Tin, Chemical elements Post-transition metals Native element minerals Chemical elements with body-centered tetragonal structure
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, the so-called " tin cry" can be heard as a result of twinning in tin crystals; this trait is shared by indium, cadmium, zinc, and mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
in the solid state.
Pure tin after solidifying presents a mirror-like appearance similar to most metals. In most tin alloys (such as pewter) the metal solidifies with a dull gray color.
Tin is a post-transition metal in group 14 of the periodic table of elements. It is obtained chiefly from the mineral cassiterite
Cassiterite is a tin oxide mineral, SnO2. It is generally opaque, but it is translucent in thin crystals. Its luster and multiple crystal faces produce a desirable gem. Cassiterite was the chief tin ore throughout ancient history and remains t ...
, which contains stannic oxide
Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin ...
, . Tin shows a chemical similarity to both of its neighbors in group 14, germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundant element on Earth and has, with 10 stable isotopes, the largest number of stable isotopes in the periodic table, thanks to its magic number of protons.
It has two main allotropes: at room temperature, the stable allotrope is β-tin, a silvery-white, malleable metal; at low temperatures it is less dense grey α-tin, which has the diamond cubic structure. Metallic tin does not easily oxidize in air and water.
The first tin alloy used on a large scale was bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such ...
, made of tin and copper, from as early as 3000 BC. After 600 BC, pure metallic tin was produced. Pewter, which is an alloy of 85–90% tin with the remainder commonly consisting of copper, antimony, bismuth, and sometimes lead and silver, has been used for flatware since the Bronze Age. In modern times, tin is used in many alloys, most notably tin / lead soft solders, which are typically 60% or more tin, and in the manufacture of transparent, electrically conducting films of indium tin oxide in optoelectronic applications. Another large application is corrosion-resistant tin plating
Tinning is the process of thinly coating sheets of wrought iron or steel with tin, and the resulting product is known as tinplate. The term is also widely used for the different process of coating a metal with solder before soldering.
It is mos ...
of steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
. Because of the low toxicity of inorganic tin, tin-plated steel is widely used for food packaging as tin cans
A steel can, tin can, tin (especially in British English, Australian English, Canadian English and South African English),
steel packaging, or can is a container for the distribution or storage of goods, made of thin metal. Many cans ...
. Some organotin compounds can be extremely toxic.
Characteristics
Physical
 Tin is a soft, malleable, ductile and highly crystalline silvery-white metal. When a bar of tin is bent a crackling sound known as the " tin cry" can be heard from the twinning of the crystals. Tin melts at about the lowest in group 14. The melting point is further lowered to for 11 nm particles.
β-tin, the metallic form or white tin, has BCT structure and is stable at and above room temperature and is malleable. α-tin, the nonmetallic form or gray tin, is stable below and is brittle. α-tin has a diamond cubic crystal structure, similar to diamond, silicon or
Tin is a soft, malleable, ductile and highly crystalline silvery-white metal. When a bar of tin is bent a crackling sound known as the " tin cry" can be heard from the twinning of the crystals. Tin melts at about the lowest in group 14. The melting point is further lowered to for 11 nm particles.
β-tin, the metallic form or white tin, has BCT structure and is stable at and above room temperature and is malleable. α-tin, the nonmetallic form or gray tin, is stable below and is brittle. α-tin has a diamond cubic crystal structure, similar to diamond, silicon or germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
. α-tin has no metallic properties, because its atoms form a covalent structure in which electrons cannot move freely. α-tin is a dull-gray powdery material with no common uses other than specialized semiconductor applications. γ-tin and σ-tin exist at temperatures above and pressures above several GPa.
In cold conditions β-tin tends to transform spontaneously into α-tin, a phenomenon known as " tin pest" or "tin disease". Some unverifiable sources also say that, during Napoleon
Napoleon Bonaparte ; it, Napoleone Bonaparte, ; co, Napulione Buonaparte. (born Napoleone Buonaparte; 15 August 1769 – 5 May 1821), later known by his regnal name Napoleon I, was a French military commander and political leader who ...
's Russian campaign of 1812, the temperatures became so cold that the tin buttons on the soldiers' uniforms disintegrated over time, contributing to the defeat of the Grande Armée
''La Grande Armée'' (; ) was the main military component of the French Imperial Army commanded by Emperor Napoleon Bonaparte during the Napoleonic Wars. From 1804 to 1808, it won a series of military victories that allowed the French Empi ...
, a persistent legend.
The α-β transformation temperature is , but impurities (e.g. Al, Zn, etc.) lower it well below . With the addition of antimony or bismuth the transformation might not occur at all, increasing durability.
Commercial grades of tin (99.8% tin content) resist transformation because of the inhibiting effect of small amounts of bismuth, antimony, lead, and silver present as impurities. Alloying elements such as copper, antimony, bismuth, cadmium, and silver increase the hardness of tin. Tin easily forms hard, brittle intermetallic phases that are typically undesirable. It does not mix into a solution with most metals and elements so tin does not have much solid solubility. Tin mixes well with bismuth, gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
, lead, thallium and zinc forming simple eutectic systems.
Tin becomes a superconductor below 3.72 K and was one of the first superconductors to be studied. The Meissner effect, one of the characteristic features of superconductors, was first discovered in superconducting tin crystals.
Chemical
Tin resists corrosion from water, but can be corroded byacid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s and alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
s. Tin can be highly polished and is used as a protective coat for other metals, a protective oxide ( passivation) layer prevents further oxidation. Tin acts as a catalyst triggering a chemical reaction of a solution containing oxygen and helps to increase the speed of the chemical reaction that results.
Isotopes
Tin has tenstable isotopes
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundanc ...
, the greatest number of any element. The isotopes of tin have atomic masses of 112, 114, 115, 116, 117, 118, 119, 120, 122, and 124. 120Sn makes up almost a third of all tin; 118Sn, and 116Sn are also common, while 115Sn is the least common stable isotope. The isotopes with even mass numbers have no nuclear spin
In atomic physics, the spin quantum number is a quantum number (designated ) which describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. The phrase was originally used to describe th ...
, while those with odd mass numbers have a spin of 1/2. Tin is among the easiest elements to detect and analyze by NMR spectroscopy which relies on molecular weight and its chemical shifts are referenced against .Only H, F, P, Tl and Xe are easier to use NMR analysis with for samples containing isotopes at their natural abundance. The large number of stable isotopes is thought to be a direct result of tin having the atomic number 50, a " magic number" in nuclear physics. Of the stable isotopes Tin-115 has a high capture cross section for fast neutron energies at 30 Barns. Two other isotopes Tin-117 ranks next with a cross section of 2.3 Barn while isotope Tin-119 has a slightly smaller cross section of 2.2 Barn. Before these cross sections were well known it was proposed to use Tin-Lead solder as a reactor coolant for fast reactors because of its low melting point. Current studies are for Lead or Lead-Bismuth reactor coolants because both heavy metals are nearly transparent to fast neutrons with very low capture cross sections. In order to use a Tin or Tin-Lead coolant the Tin would first have to go through isotopes separation to remove the 115, 117 and 119 isotopes from the material. Combined these three isotopes make up about 17% of the entire mass of natural Tin but represent nearly all of the capture cross section. Of the remaining seven isotopes Tin-112 has a capture cross section of 1 Barn. The other six isotopes forming 82.7% of all Tin have capture cross sections of 0.3 Barn or less making them effectively transparent to neutrons like Lead and Bismuth.
Tin has 31 unstable isotopes, ranging in mass number from 99 to 139. The unstable tin isotopes have a half-life of less than a year except 126Sn which has a half-life of 230,000 years.
100Sn and 132Sn are two of the few nuclide
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by Truman ...
s with a " doubly magic" nucleus which despite being unstable, as they have very uneven neutron–proton ratio
The neutron–proton ratio (N/Z ratio or nuclear ratio) of an atomic nucleus is the ratio of its number of neutrons to its number of protons. Among stable nuclei and naturally occurring nuclei, this ratio generally increases with increasing atomi ...
s, are the endpoints beyond which tin isotopes lighter than 100Sn and heavier than 132Sn are much less stable. Another 30 metastable isomers
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have ha ...
have been identified for tin isotopes between 111 and 131, the most stable being 121mSn, with a half-life of 43.9 years.
The relative differences in the number of tin's stable isotopes can be explained by how they are formed during stellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
. 116Sn through 120Sn are formed in the ''s''-process (slow neutron capture) in most star
A star is an astronomical object comprising a luminous spheroid of plasma (physics), plasma held together by its gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked ...
s which leads to them being the most common tin isotopes, while 122Sn and 124Sn are only formed in the ''r''-process (rapid neutron capture) in supernovae
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when a ...
and are less common. Tin isotopes 117Sn through 120Sn are also produced in the ''r''-process. 112Sn, 114Sn, and 115Sn, cannot be made in significant amounts in the ''s''- or ''r''-processes and are among the p-nuclei whose origins are not well understood. Some ideas about for their formation include proton capture and photodisintegration, 115Sn might be partially produced in the ''s''-process both directly and as the daughter of long-lived 115In.
Etymology
The word ''tin'' is shared among Germanic languages and can be traced back to reconstructed Proto-Germanic ;cognate
In historical linguistics, cognates or lexical cognates are sets of words in different languages that have been inherited in direct descent from an etymology, etymological ancestor in a proto-language, common parent language. Because language c ...
s include German , Swedish
Swedish or ' may refer to:
Anything from or related to Sweden, a country in Northern Europe. Or, specifically:
* Swedish language, a North Germanic language spoken primarily in Sweden and Finland
** Swedish alphabet, the official alphabet used by ...
and Dutch . It is not found in other branches of Indo-European, except by borrowing from Germanic (e.g., Irish from English).
The Latin name for tin, , originally meant an alloy of silver and lead, and came to mean 'tin' in the fourth century—the earlier Latin word for it was , or "white lead". apparently came from an earlier (meaning the same substance), the origin of the Romance and Celtic
Celtic, Celtics or Keltic may refer to:
Language and ethnicity
*pertaining to Celts, a collection of Indo-European peoples in Europe and Anatolia
**Celts (modern)
*Celtic languages
**Proto-Celtic language
* Celtic music
*Celtic nations
Sports Fo ...
terms for ''tin'', such as French
French (french: français(e), link=no) may refer to:
* Something of, from, or related to France
** French language, which originated in France, and its various dialects and accents
** French people, a nation and ethnic group identified with Franc ...
, Spanish , Italian , and Irish . The origin of / is unknown; it may be pre- Indo-European.
The suggests instead that came from Cornish , and is evidence that Cornwall in the first centuries AD was the main source of tin.
History
 Tin extraction and use can be dated to the beginnings of the Bronze Age around 3000 BC, when it was observed that copper objects formed of polymetallic
Tin extraction and use can be dated to the beginnings of the Bronze Age around 3000 BC, when it was observed that copper objects formed of polymetallic ores
Ore is natural Rock (geology), rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Ret ...
with different metal contents had different physical properties. The earliest bronze objects had a tin or arsenic content of less than 2% and are believed to be the result of unintentional alloying due to trace metal content in the copper ore. The addition of a second metal to copper increases its hardness, lowers the melting temperature, and improves the casting process by producing a more fluid melt that cools to a denser, less spongy metal. This was an important innovation that allowed for the much more complex shapes cast in closed molds of the Bronze Age. Arsenical bronze objects appear first in the Near East where arsenic is commonly found with copper ore, but the health risks were quickly realized and the quest for sources of the much less hazardous tin ores began early in the Bronze Age. This created the demand for rare tin metal and formed a trade network that linked the distant sources of tin to the markets of Bronze Age cultures.
Cassiterite
Cassiterite is a tin oxide mineral, SnO2. It is generally opaque, but it is translucent in thin crystals. Its luster and multiple crystal faces produce a desirable gem. Cassiterite was the chief tin ore throughout ancient history and remains t ...
(), the oxide form of tin, was most likely the original source of tin. Other tin ores are less common sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
s such as stannite
Stannite is a mineral, a sulfide of copper, iron, and tin, in the category of thiostannates. Background
The chemical formula Cu2 Fe Sn S4. Zinc commonly occurs with the iron and trace germanium may be present. Stannite is used as an ore of tin, ...
that require a more involved smelting process. Cassiterite often accumulates in alluvial channels as placer deposits
In geology, a placer deposit or placer is an accumulation of valuable minerals formed by gravity separation from a specific source rock during sedimentary processes. The name is from the Spanish word ''placer'', meaning "alluvial sand". Placer min ...
because it is harder, heavier, and more chemically resistant than the accompanying granite. Cassiterite is usually black or dark in color, and these deposits can be easily seen in river banks. Alluvial ( placer) deposits may incidentally have been collected and separated by methods similar to gold panning.
Compounds and chemistry
In the great majority of its compounds, tin has the oxidation state II or IV. Compounds containingbivalent Bivalent may refer to:
* Bivalent (chemistry), a molecule formed from two or more atoms bound together
*Bivalent (engine), an engine that can operate on two different types of fuel
*Bivalent (genetics), a pair of homologous chromosomes
*Bivalent log ...
tin are called while those containing tetravalent tin are termed .
Inorganic compounds
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
compounds are known for both oxidation states. For Sn(IV), all four halides are well known: SnF4, SnCl4, SnBr4, and SnI4. The three heavier members are volatile molecular compounds, whereas the tetrafluoride is polymeric. All four halides are known for Sn(II) also: SnF2, , SnBr2, and SnI2. All are polymeric solids. Of these eight compounds, only the iodides are colored.
Tin(II) chloride (also known as stannous chloride) is the most important commercial tin halide. Illustrating the routes to such compounds, chlorine reacts with tin metal to give SnCl4 whereas the reaction of hydrochloric acid and tin produces and hydrogen gas. Alternatively SnCl4 and Sn combine to stannous chloride by a process called comproportionation:
:SnCl4 + Sn → 2
Tin can form many oxides, sulfides, and other chalcogenide derivatives. The dioxide (cassiterite) forms when tin is heated in the presence of air. is amphoteric, which means that it dissolves in both acidic and basic solutions. Stannates with the structure []2−, like [], are also known, though the free stannic acid [] is unknown.
Sulfides of tin exist in both the +2 and +4 oxidation states: tin(II) sulfide and tin(IV) sulfide ([ osaic gold).

Hydrides
Stannane (), with tin in the +4 oxidation state, is unstable. Organotin hydrides are however well known, e.g. tributyltin hydride (Sn(C4H9)3H). These compound release transienttributyl tin
Tributyltin (TBT) is an umbrella term for a class of organotin compounds which contain the (C4H9)3 Sn group, with a prominent example being tributyltin oxide. For 40 years TBT was used as a biocide in anti-fouling paint, commonly known as botto ...
radicals, which are rare examples of compounds of tin(III).
Organotin compounds
Organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by ...
compounds, sometimes called stannanes, are chemical compounds with tin–carbon bonds. Of the tin compounds, the organic derivatives are commercially the most useful. Some organotin compounds are highly toxic and have been used as biocide
A biocide is defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism. The US Environmental Protection Agency (EPA) uses a slig ...
s. The first organotin compound to be reported was diethyltin diiodide ((C2H5)2SnI2), reported by Edward Frankland in 1849.
Most organotin compounds are colorless liquids or solids that are stable to air and water. They adopt tetrahedral geometry. Tetraalkyl- and tetraaryltin compounds can be prepared using Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
s:
: + 4 RMgBr → + 4 MgBrCl
The mixed halide-alkyls, which are more common and more important commercially than the tetraorgano derivatives, are prepared by redistribution reaction In chemistry, redistribution usually refers to the exchange of anionic ligands bonded to metal and metalloid centers. The conversion does not involve redox, in contrast to disproportionation reactions. Some useful redistribution reactions are condu ...
s:
: + → 2 R2
Divalent organotin compounds are uncommon, although more common than related divalent organogermanium and organosilicon
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic co ...
compounds. The greater stabilization enjoyed by Sn(II) is attributed to the " inert pair effect". Organotin(II) compounds include both stannylenes (formula: R2Sn, as seen for singlet carbenes) and distannylenes (R4Sn2), which are roughly equivalent to alkenes. Both classes exhibit unusual reactions.
Occurrence
 Tin is generated via the long ''s''-process in low-to-medium mass stars (with masses of 0.6 to 10 times that of the Sun), and finally by beta decay of the heavy isotopes of indium.
Tin is the 49th most abundant element in
Tin is generated via the long ''s''-process in low-to-medium mass stars (with masses of 0.6 to 10 times that of the Sun), and finally by beta decay of the heavy isotopes of indium.
Tin is the 49th most abundant element in Earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
, representing 2 ppm compared with 75 ppm for zinc, 50 ppm for copper, and 14 ppm for lead.
Tin does not occur as the native element but must be extracted from various ores. Cassiterite
Cassiterite is a tin oxide mineral, SnO2. It is generally opaque, but it is translucent in thin crystals. Its luster and multiple crystal faces produce a desirable gem. Cassiterite was the chief tin ore throughout ancient history and remains t ...
() is the only commercially important source of tin, although small quantities of tin are recovered from complex sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
s such as stannite
Stannite is a mineral, a sulfide of copper, iron, and tin, in the category of thiostannates. Background
The chemical formula Cu2 Fe Sn S4. Zinc commonly occurs with the iron and trace germanium may be present. Stannite is used as an ore of tin, ...
, cylindrite, franckeite
Franckeite, chemical formula Pb5Sn3Sb2S14, belongs to a family of complex sulfide minerals. Franckeite is a sulfosalt. It is closely related to cylindrite.
It was first described in 1893 for an occurrence in Chocaya, Potosí Department, Bolivia. ...
, canfieldite, and teallite. Minerals with tin are almost always associated with granite rock, usually at a level of 1% tin oxide content.
Because of the higher specific gravity of tin dioxide, about 80% of mined tin is from secondary deposits found downstream from the primary lodes. Tin is often recovered from granules washed downstream in the past and deposited in valleys or the sea. The most economical ways of mining tin are by dredging
Dredging is the excavation of material from a water environment. Possible reasons for dredging include improving existing water features; reshaping land and water features to alter drainage, navigability, and commercial use; constructing da ...
, hydraulicking
Hydraulic mining is a form of mining that uses high-pressure jets of water to dislodge rock material or move sediment.Paul W. Thrush, ''A Dictionary of Mining, Mineral, and Related Terms'', US Bureau of Mines, 1968, p.560. In the placer mining of ...
, or open pits
Open-pit mining, also known as open-cast or open-cut mining and in larger contexts mega-mining, is a surface mining technique of extracting rock or minerals from the earth from an open-air pit, sometimes known as a borrow.
This form of mining ...
. Most of the world's tin is produced from placer deposits, which can contain as little as 0.015% tin.
About 253,000 tonnes of tin were mined in 2011, mostly in China (110,000 t), Indonesia (51,000 t), Peru (34,600 t), Bolivia (20,700 t) and Brazil (12,000 t). Estimates of tin production have historically varied with the market and mining technology. It is estimated that, at current consumption rates and technologies, the Earth will run out of mine-able tin in 40 years. In 2006 Lester Brown suggested tin could run out within 20 years based on conservative estimates of 2% annual growth.
Scrap tin is an important source of the metal. Recovery of tin through recycling is increasing rapidly. Whereas the United States has neither mined (since 1993) nor smelted (since 1989) tin, it was the largest secondary producer, recycling nearly 14,000 tonnes in 2006.
New deposits are reported in Mongolia, and in 2009, new deposits of tin were discovered in Colombia.
Production
Tin is produced by carbothermic reduction of the oxide ore with carbon or coke. Both reverberatory furnace and electric furnace can be used.Mining and smelting
Industry
The ten largest companies produced most of the world's tin in 2007. Most of the world's tin is traded on LME, from 8 countries, under 17 brands. International Tin Council was established in 1947 to control the price of tin. It collapsed in 1985. In 1984, ''Association of Tin Producing Countries'' was created, with Australia, Bolivia, Indonesia, Malaysia, Nigeria, Thailand, and Zaire as members.Price and exchanges
 Tin is unique among mineral commodities because of the complex agreements between producer countries and consumer countries dating back to 1921. Earlier agreements tended to be somewhat informal and led to the "First International Tin Agreement" in 1956, the first of a series that effectively collapsed in 1985. Through these agreements, the International Tin Council (ITC) had a considerable effect on tin prices. ITC supported the price of tin during periods of low prices by buying tin for its buffer stockpile and was able to restrain the price during periods of high prices by selling from the stockpile. This was an anti-free-market approach, designed to assure a sufficient flow of tin to consumer countries and a profit for producer countries. However, the buffer stockpile was not sufficiently large, and during most of those 29 years tin prices rose, sometimes sharply, especially from 1973 through 1980 when rampant inflation plagued many world economies.
During the late 1970s and early 1980s, the U.S. reduced its strategic tin stockpile, partly to take advantage of historically high tin prices. The 1981–82 recession damaged the tin industry. Tin consumption declined dramatically. ITC was able to avoid truly steep declines through accelerated buying for its buffer stockpile; this activity required extensive borrowing. ITC continued to borrow until late 1985 when it reached its credit limit. Immediately, a major "tin crisis" ensued — tin was delisted from trading on the London Metal Exchange for about three years. ITC dissolved soon afterward, and the price of tin, now in a free-market environment, fell to $4 per pound and remained around that level through the 1990s. The price increased again by 2010 with a rebound in consumption following the 2007–2008 economic crisis, accompanying restocking and continued growth in consumption.
Tin is unique among mineral commodities because of the complex agreements between producer countries and consumer countries dating back to 1921. Earlier agreements tended to be somewhat informal and led to the "First International Tin Agreement" in 1956, the first of a series that effectively collapsed in 1985. Through these agreements, the International Tin Council (ITC) had a considerable effect on tin prices. ITC supported the price of tin during periods of low prices by buying tin for its buffer stockpile and was able to restrain the price during periods of high prices by selling from the stockpile. This was an anti-free-market approach, designed to assure a sufficient flow of tin to consumer countries and a profit for producer countries. However, the buffer stockpile was not sufficiently large, and during most of those 29 years tin prices rose, sometimes sharply, especially from 1973 through 1980 when rampant inflation plagued many world economies.
During the late 1970s and early 1980s, the U.S. reduced its strategic tin stockpile, partly to take advantage of historically high tin prices. The 1981–82 recession damaged the tin industry. Tin consumption declined dramatically. ITC was able to avoid truly steep declines through accelerated buying for its buffer stockpile; this activity required extensive borrowing. ITC continued to borrow until late 1985 when it reached its credit limit. Immediately, a major "tin crisis" ensued — tin was delisted from trading on the London Metal Exchange for about three years. ITC dissolved soon afterward, and the price of tin, now in a free-market environment, fell to $4 per pound and remained around that level through the 1990s. The price increased again by 2010 with a rebound in consumption following the 2007–2008 economic crisis, accompanying restocking and continued growth in consumption.
 London Metal Exchange (LME) is tin's principal trading site. Other tin contract markets are Kuala Lumpur Tin Market (KLTM) and Indonesia Tin Exchange (INATIN).
Due to factors involved in the
London Metal Exchange (LME) is tin's principal trading site. Other tin contract markets are Kuala Lumpur Tin Market (KLTM) and Indonesia Tin Exchange (INATIN).
Due to factors involved in the 2021 global supply chain crisis
1 (one, unit, unity) is a number representing a single or the only entity. 1 is also a numerical digit and represents a single unit of counting or measurement. For example, a line segment of ''unit length'' is a line segment of length 1 ...
, tin prices almost doubled between 2020—21 and have had their largest annual rise in over 30 years. The International Tin Association estimated that global refined tin consumption will grow 7.2 percent in 2021, after losing 1.6 percent in 2020 as the COVID-19 pandemic disrupted global manufacturing industries.
Applications
In 2018, just under half of all tin produced was used in solder. The rest was divided between tin plating, tin chemicals, brass and bronze alloys, and niche uses.Solder
 Tin has long been used in alloys with lead as solder, in amounts of 5 to 70% w/w. Tin with lead forms a eutectic mixture at the weight proportion of 61.9% tin and 38.1% lead (the atomic proportion: 73.9% tin and 26.1% lead), with melting temperature of 183 °C (361.4 °F). Such solders are primarily used for joining pipes or electric circuits. Since the European Union Waste Electrical and Electronic Equipment Directive (WEEE Directive) and Restriction of Hazardous Substances Directive came into effect on 1 July 2006, the lead content in such alloys has decreased. While lead exposure is associated with serious health problems, lead-free solder is not without its challenges, including a higher melting point, and the formation of tin whiskers that cause electrical problems. Tin pest can occur in lead-free solders, leading to loss of the soldered joint. Replacement alloys are being found, but the problems of joint integrity remain.
Tin has long been used in alloys with lead as solder, in amounts of 5 to 70% w/w. Tin with lead forms a eutectic mixture at the weight proportion of 61.9% tin and 38.1% lead (the atomic proportion: 73.9% tin and 26.1% lead), with melting temperature of 183 °C (361.4 °F). Such solders are primarily used for joining pipes or electric circuits. Since the European Union Waste Electrical and Electronic Equipment Directive (WEEE Directive) and Restriction of Hazardous Substances Directive came into effect on 1 July 2006, the lead content in such alloys has decreased. While lead exposure is associated with serious health problems, lead-free solder is not without its challenges, including a higher melting point, and the formation of tin whiskers that cause electrical problems. Tin pest can occur in lead-free solders, leading to loss of the soldered joint. Replacement alloys are being found, but the problems of joint integrity remain.
Tin plating
 Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. Tin-plated (or tinning) steel containers is widely used for
Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. Tin-plated (or tinning) steel containers is widely used for food preservation
Food preservation includes processes that make food more resistant to microorganism growth and slow the oxidation of fats. This slows down the decomposition and rancidification process. Food preservation may also include processes that inhibit ...
, and this forms a large part of the market for metallic tin. A tinplate canister for preserving food was first manufactured in London in 1812. Speakers of British English call such containers "tins", while speakers of American English call them " cans" or "tin cans". One derivation of such use is the slang term "tinnie
The slang or colloquial term tinnie or tinny has a variety of meanings, generally derived from some association with the metal tin, or aluminium foil which has a loose allusion to tin.
"Tinnie" is the common term for a commemorative medal mad ...
" or "tinny", meaning "can of beer" in Australia. The tin whistle is so called because it was mass-produced first in tin-plated steel.
Copper cooking vessels such as saucepans and frying pans are frequently lined with a thin plating of tin, by electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be ...
or by traditional chemical methods, since use of copper cookware with acidic foods can be toxic.
Specialized alloys

 Tin in combination with other elements forms a wide variety of useful alloys. Tin is most commonly alloyed with copper. Pewter is 85–99% tin; bearing metal has a high percentage of tin as well.
Tin in combination with other elements forms a wide variety of useful alloys. Tin is most commonly alloyed with copper. Pewter is 85–99% tin; bearing metal has a high percentage of tin as well. Bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such ...
is mostly copper with 12% tin, while the addition of phosphorus yields phosphor bronze. Bell metal
Bell metal or bell bronze is an alloy used for making bells and related instruments, such as cymbals. It is a form of bronze with a higher tin content, usually in approximately a 4:1 ratio of copper to tin (typically, 78% copper, 22% tin by mas ...
is also a copper–tin alloy, containing 22% tin. Tin has sometimes been used in coinage; it once formed a single-digit percentage (usually five percent or less) of American and Canadian pennies. Because copper is often the major metal in such coins, sometimes including zinc, these could be called bronze, or brass alloys.
The niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
–tin compound Nb3Sn is commercially used in coils of superconducting magnet
A superconducting magnet is an electromagnet made from coils of superconducting wire. They must be cooled to cryogenic temperatures during operation. In its superconducting state the wire has no electrical resistance and therefore can conduct mu ...
s for its high critical temperature
Critical or Critically may refer to:
*Critical, or critical but stable, medical states
**Critical, or intensive care medicine
*Critical juncture, a discontinuous change studied in the social sciences.
*Critical Software, a company specializing in ...
(18 K) and critical magnetic field (25 T). A superconducting magnet weighing as little as two kilogram
The kilogram (also kilogramme) is the unit of mass in the International System of Units (SI), having the unit symbol kg. It is a widely used measure in science, engineering and commerce worldwide, and is often simply called a kilo colloquially ...
s is capable of producing the magnetic field of a conventional electromagnet weighing tons.
A small percentage of tin is added to zirconium alloys for the cladding of nuclear fuel.
Most metal pipes in a pipe organ
The pipe organ is a musical instrument that produces sound by driving pressurized air (called ''wind'') through the organ pipes selected from a keyboard. Because each pipe produces a single pitch, the pipes are provided in sets called ''ranks ...
are of a tin/lead alloy, with 50/50 as the most common composition. The proportion of tin in the pipe defines the pipe's tone, since tin has a desirable tonal resonance. When a tin/lead alloy cools, the lead phase solidifies first, then when the eutectic temperature is reached, the remaining liquid forms the layered tin/lead eutectic structure, which is shiny; contrast with the lead phase produces a mottled or spotted effect. This metal alloy is referred to as spotted metal. Major advantages of using tin for pipes include its appearance, workability, and resistance to corrosion.
Optoelectronics
The oxides of indium and tin are electrically conductive and transparent, and are used to make transparent electrically conducting films with applications in optoelectronics devices such as liquid crystal displays.Other applications
 Punched tin-plated steel, also called pierced tin, is an artisan technique originating in central Europe for creating functional and decorative housewares. Decorative piercing designs exist in a wide variety, based on local tradition and the artisan. Punched tin lanterns are the most common application of this artisan technique. The light of a candle shining through the pierced design creates a decorative light pattern in the room where it sits. Lanterns and other punched tin articles were created in the New World from the earliest European settlement. A well-known example is the Revere lantern, named after Paul Revere.
Before the modern era, in some areas of the Alps, a goat or sheep's horn would be sharpened and a tin panel would be punched out using the alphabet and numbers from one to nine. This learning tool was known appropriately as "the horn". Modern reproductions are decorated with such motifs as hearts and tulips.
In America, pie safes and food safes were in use in the days before refrigeration. These were wooden cupboards of various styles and sizes – either floor standing or hanging cupboards meant to discourage vermin and insects and to keep dust from perishable foodstuffs. These cabinets had tinplate inserts in the doors and sometimes in the sides, punched out by the homeowner, cabinetmaker, or a tinsmith in varying designs to allow for air circulation while excluding flies. Modern reproductions of these articles remain popular in North America.
Window glass is most often made by floating molten glass on molten tin (
Punched tin-plated steel, also called pierced tin, is an artisan technique originating in central Europe for creating functional and decorative housewares. Decorative piercing designs exist in a wide variety, based on local tradition and the artisan. Punched tin lanterns are the most common application of this artisan technique. The light of a candle shining through the pierced design creates a decorative light pattern in the room where it sits. Lanterns and other punched tin articles were created in the New World from the earliest European settlement. A well-known example is the Revere lantern, named after Paul Revere.
Before the modern era, in some areas of the Alps, a goat or sheep's horn would be sharpened and a tin panel would be punched out using the alphabet and numbers from one to nine. This learning tool was known appropriately as "the horn". Modern reproductions are decorated with such motifs as hearts and tulips.
In America, pie safes and food safes were in use in the days before refrigeration. These were wooden cupboards of various styles and sizes – either floor standing or hanging cupboards meant to discourage vermin and insects and to keep dust from perishable foodstuffs. These cabinets had tinplate inserts in the doors and sometimes in the sides, punched out by the homeowner, cabinetmaker, or a tinsmith in varying designs to allow for air circulation while excluding flies. Modern reproductions of these articles remain popular in North America.
Window glass is most often made by floating molten glass on molten tin (float glass
Float glass is a sheet of glass made by floating molten glass on a bed of molten metal, typically tin, although lead and other various low- melting-point alloys were used in the past. This method gives the sheet uniform thickness and very flat sur ...
), resulting in a flat and flawless surface. This is also called the "Pilkington process
Float glass is a sheet of glass made by floating molten glass on a bed of molten metal, typically tin, although lead and other various low-melting point, melting-point alloys were used in the past. This method gives the sheet uniform thickness and ...
".
Tin is used as a negative electrode in advanced Li-ion batteries. Its application is somewhat limited by the fact that some tin surfaces catalyze decomposition of carbonate-based electrolytes used in Li-ion batteries.
Tin(II) fluoride
Tin(II) fluoride, commonly referred to commercially as stannous fluoride (from Latin ', 'tin'), is a chemical compound with the formula SnF2. It is a colourless solid used as an ingredient in toothpastes.
Oral health benefits
Stannous fluoride wa ...
is added to some dental care products as stannous fluoride (SnF2). Tin(II) fluoride
Tin(II) fluoride, commonly referred to commercially as stannous fluoride (from Latin ', 'tin'), is a chemical compound with the formula SnF2. It is a colourless solid used as an ingredient in toothpastes.
Oral health benefits
Stannous fluoride wa ...
can be mixed with calcium abrasives while the more common sodium fluoride gradually becomes biologically inactive in the presence of calcium compounds. It has also been shown to be more effective than sodium fluoride in controlling gingivitis.
Tin is used as a target to create laser-induced plasmas that act as the light source for extreme ultraviolet lithography
Extreme ultraviolet lithography (also known as EUV or EUVL) is an optical lithography technology used in steppers, machines that make integrated circuits (ICs) for computers and other electronic devices. It uses a range of extreme ultraviolet (EUV) ...
.
Organotin compounds
The organotin compounds are most heavily used. Worldwide industrial production probably exceeds 50,000 tonnes.PVC stabilizers
The major commercial application of organotin compounds is in the stabilization of PVC plastics. In the absence of such stabilizers, PVC would rapidly degrade under heat, light, and atmospheric oxygen, resulting in discolored, brittle products. Tin scavenges labile chloride ions (Cl−), which would otherwise strip HCl from the plastic material. Typical tin compounds are carboxylic acid derivatives of dibutyltin dichloride, such as the dilaurate
Lauric acid, systematically dodecanoic acid, is a saturated fatty acid with a 12-carbon atom chain, thus having many properties of medium-chain fatty acids. It is a bright white, powdery solid with a faint odor of bay oil or soap. The salts and ...
.
Biocides
Some organotin compounds are relatively toxic, with both advantages and problems. They are used for biocidal properties asfungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, ...
s, pesticide
Pesticides are substances that are meant to control pests. This includes herbicide, insecticide, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, microbicide, fungicide, and lampri ...
s, algaecides, wood preservatives, and antifouling agent
Biofouling or biological fouling is the accumulation of microorganisms, plants, algae, or small animals where it is not wanted on surfaces such as ship and submarine hulls, devices such as water inlets, pipework, grates, ponds, and rivers that ...
s. Tributyltin oxide is used as a wood preservative. Tributyltin is also used for various industrial purposes such as slime control in paper mills and disinfection of circulating industrial cooling waters. Tributyltin was used as additive for ship paint to prevent growth of fouling organisms on ships, with use declining after organotin compounds were recognized as persistent organic pollutants with high toxicity for some marine organisms (the dog whelk, for example). The EU banned the use of organotin compounds in 2003, while concerns over the toxicity of these compounds to marine life and damage to the reproduction and growth of some marine species (some reports describe biological effects to marine life at a concentration of 1 nanogram per liter) have led to a worldwide ban by the International Maritime Organization
The International Maritime Organization (IMO, French: ''Organisation maritime internationale'') is a specialised agency of the United Nations responsible for regulating shipping. The IMO was established following agreement at a UN conference ...
. Many nations now restrict the use of organotin compounds to vessels greater than long. The persistence of tributyltin in the aquatic environment is dependent upon the nature of the ecosystem. Because of this persistence and its use as an additive in ship paint, high concentrations of tributyltin have been found in marine sediments located near naval docks. Tributyltin has been used as a biomarker for imposex in neograstropods, with at least 82 known species. With the high levels of TBT in the local inshore areas, due to shipping activities, the shellfish had an adverse effect. Imposex is the imposition of male sexual characteristics on female specimens where they grow a penis and a pallial vas deferens. A high level of TBT can damage mammalian endocrine glands, reproductive
The reproductive system of an organism, also known as the genital system, is the biological system made up of all the anatomical organs involved in sexual reproduction. Many non-living substances such as fluids, hormones, and pheromones are als ...
and central nervous systems, bone structure and gastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organ (biology), organs of the digestive syste ...
. Not only does tributyltin affect mammals, it affects sea otters, whales, dolphins, and humans.
Organic chemistry
Some tinreagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s are useful in organic chemistry. In the largest application, stannous chloride is a common reducing agent for the conversion of nitro and oxime groups to amines. The Stille reaction couples organotin compounds with organic halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s or pseudohalides.
Li-ion batteries
Tin forms several inter-metallic phases with lithium metal, making it a potentially attractive material for battery applications. Large volumetric expansion of tin upon alloying with lithium and instability of the tin-organic electrolyte interface at low electrochemical potentials are the greatest challenges to employment in commercial cells. Tin inter-metallic compound with cobalt and carbon was implemented by Sony in its Nexelion cells released in the late 2000s. The composition of the active material is approximately Sn0.3Co0.4C0.3. Research showed that only some crystalline facets of tetragonal (beta) Sn are responsible for undesirable electrochemical activity.Precautions
Cases of poisoning from tin metal, its oxides, and its salts are almost unknown. On the other hand, certainorganotin compound
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by ...
s are almost as toxic as cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
.Graf, G. G. (2000) "Tin, Tin Alloys, and Tin Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005 Wiley-VCH, Weinheim
Exposure to tin in the workplace can occur by inhalation, skin contact, and eye contact. The US Occupational Safety and Health Administration (OSHA) set the permissible exposure limit for tin exposure in the workplace as 2 mg/m3 over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) determined a recommended exposure limit (REL) of 2 mg/m3 over an 8-hour workday. At levels of 100 mg/m3, tin is IDLH, immediately dangerous to life and health.
See also
* Cassiterides (the mythical Tin Islands) * List of countries by tin production * Stannary * Terne * Tin pest * Tin mining in Britain * Tinning * Whisker (metallurgy) (tin whiskers)Notes
References
Bibliography
* * * * * * *External links
Tin
at ''The Periodic Table of Videos'' (University of Nottingham)
Theodore Gray's Wooden Periodic Table Table
Tin samples and castings
Tin (USD cents per kg)
{{Authority control Tin, Chemical elements Post-transition metals Native element minerals Chemical elements with body-centered tetragonal structure