|
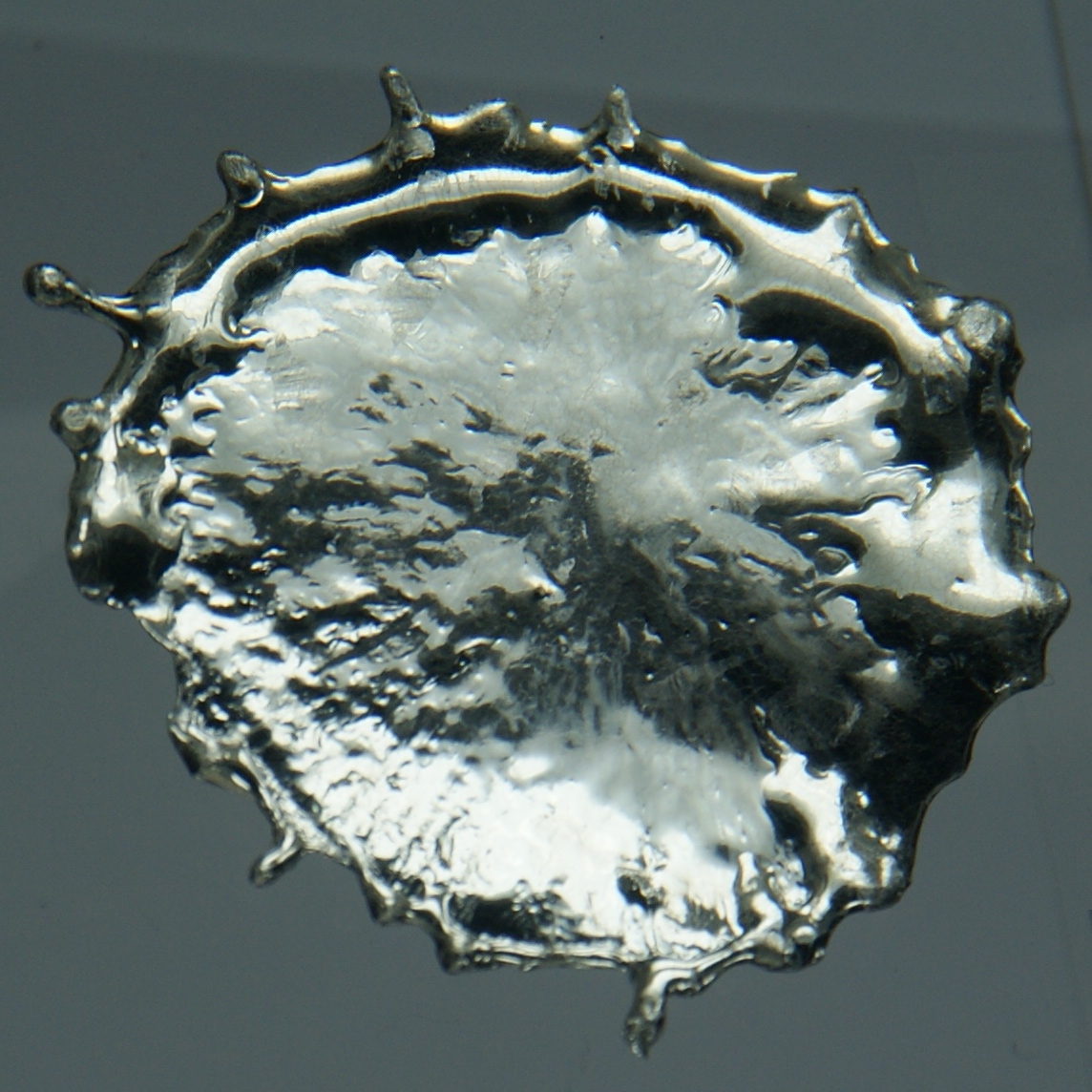
Stannite
Stannite is a mineral, a sulfide of copper, iron, and tin, in the category of thiostannates. Background The chemical formula Cu2 Fe Sn S4. Zinc commonly occurs with the iron and trace germanium may be present. Stannite is used as an ore of tin, consisting of approximately 28% tin, 13% iron, 30% copper, 30% sulfur by mass. It is found in tin-bearing, hydrothermal vein deposits occurring with chalcopyrite, sphalerite, tetrahedrite, arsenopyrite, pyrite, cassiterite Cassiterite is a tin oxide mineral, SnO2. It is generally opaque, but it is translucent in thin crystals. Its luster and multiple crystal faces produce a desirable gem. Cassiterite was the chief tin ore throughout ancient history and remains t ..., and wolframite. It is also known as ''bell metal ore'' as tin is an important constituent of bell-metal. It is thought the exploitation of tin deposits in Cornwall led to an expansion in bell founding. The name comes from the Latin for tin: ''stannum''. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Kesterite
Kësterite is a sulfide mineral with a chemical formula of . In its lattice structure, zinc and iron atoms share the same lattice sites. Kesterite is the Zn-rich variety whereas the Zn-poor form is called ferrokesterite or stannite. Owing to their similarity, kesterite is sometimes called isostannite. The synthetic form of kesterite is abbreviated as CZTS (from copper zinc tin sulfide). The name kesterite is sometimes extended to include this synthetic material and also CZTSe, which contains selenium instead of sulfur. Occurrence Kesterite was first described in 1958 in regard to an occurrence in the Kester deposit (and the associated locality) in Ynnakh Mountain, Yana basin, Yakutia, Russia, where it was discovered. It is usually found in quartz-sulfide hydrothermal veins associated with tin ore deposits. Associated minerals include arsenopyrite, stannoidite, chalcopyrite, chalcocite, sphalerite and tennantite. Stannite and kesterite occur together in the Ivigtut cryoli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Thiostannates
Sulfidostannates, or thiostannates are chemical compounds containing anions composed of tin linked with sulfur. They can be considered as stannates with sulfur substituting for oxygen. Related compounds include the thiosilicates, and thiogermannates, and by varying the chalcogen: selenostannates, and tellurostannates. Oxothiostannates have oxygen in addition to sulfur. Thiostannates can be classed as chalcogenidometalates, thiometallates, chalcogenidotetrelates, thiotetrelates, and chalcogenidostannates. Tin is almost always in the +4 oxidation state in thiostannates, although a couple of mixed sulfides in the +2 state are known, Some thiostannate minerals are known. In nature the tin can be partly replaced by arsenic, germanium, antimony or indium. Many thiostannate minerals contain copper, silver or lead. In the field of mineralogy, these compound can be termed sulfostannates or sulphostannates. Different cluster anions are known: nS4sup>4–, nS3sup>2–, n2S5sup>2–, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Tin Minerals
Tin is a chemical element with the Chemical symbol, symbol Sn (from la, :la:Stannum, stannum) and atomic number 50. Tin is a silvery-coloured metal. Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, the so-called "tin cry" can be heard as a result of Crystal twinning, twinning in tin crystals; this trait is shared by indium, cadmium, zinc, and Mercury (element), mercury in the solid state. Pure tin after solidifying presents a mirror-like appearance similar to most metals. In most tin alloys (such as pewter) the metal solidifies with a dull gray color. Tin is a post-transition metal in Carbon group, group 14 of the Periodic table, periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains Tin(IV) oxide, stannic oxide, . Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Sulfide Mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, the arsenides, the antimonides, the bismuthinides, the sulfarsenides and the sulfosalts.http://www.minerals.net/mineral/sort-met.hod/group/sulfgrp.htm Minerals.net Dana Classification, SulfidesKlein, Cornelis and Cornelius S. Hurlbut, Jr., 1986, ''Manual of Mineralogy'', Wiley, 20th ed., pp 269-293 Sulfide minerals are inorganic compounds. Minerals Common or important examples include: * Acanthite * Chalcocite * Bornite * Galena *Sphalerite * Chalcopyrite * Pyrrhotite * Millerite * Pentlandite * Covellite *Cinnabar * Realgar * Orpiment * Stibnite * Pyrite * Marcasite * Molybdenite Sulfarsenides: * Cobaltite * Arsenopyrite * Gersdorffite Sulfosalts: * Pyrargyrite * Proustite * Tetrahedrite * Tennantite * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Sulfide Minerals
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, the arsenides, the antimonides, the bismuthinides, the sulfarsenides and the sulfosalts.http://www.minerals.net/mineral/sort-met.hod/group/sulfgrp.htm Minerals.net Dana Classification, SulfidesKlein, Cornelis and Cornelius S. Hurlbut, Jr., 1986, ''Manual of Mineralogy'', Wiley, 20th ed., pp 269-293 Sulfide minerals are inorganic compounds. Minerals Common or important examples include: * Acanthite * Chalcocite * Bornite *Galena *Sphalerite *Chalcopyrite *Pyrrhotite * Millerite *Pentlandite * Covellite *Cinnabar * Realgar *Orpiment * Stibnite *Pyrite *Marcasite * Molybdenite Sulfarsenides: * Cobaltite *Arsenopyrite * Gersdorffite Sulfosalts: * Pyrargyrite *Proustite *Tetrahedrite * Tennantite * Enargite * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Tetragonal Minerals
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the producti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Wolframite
Wolframite is an iron, manganese, and tungstate mineral with a chemical formula of that is the intermediate between ferberite ( rich) and hübnerite ( rich). Along with scheelite, the wolframite series are the most important tungsten ore minerals. Wolframite is found in quartz veins and pegmatites associated with granitic intrusives. Associated minerals include cassiterite, scheelite, bismuth, quartz, pyrite, galena, sphalerite, and arsenopyrite. This mineral was historically found in Europe in Bohemia, Saxony, and Cornwall. China reportedly has the world's largest supply of tungsten ore with about 60%. Other producers are Spain, Canada, Portugal, Russia, Australia, Thailand, South Korea, Rwanda, Bolivia, the United States, and the Democratic Republic of the Congo. Name The name "wolframite" is derived from German "''wolf rahm''", the name given to tungsten by Johan Gottschalk Wallerius in 1747. This, in turn, derives from "''Lupi spuma''", the name Georg Agricola used f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide. Chemical properties The sulfide ion, S2−, does not exist in aqueous alkaline solutions of Na2S. Instead sulfide converts to hydrosulfide: :S2− + H2O → SH− + OH− Upon treatment with an acid, sulfide salts convert to hydrogen sulfide: :S2− + H+ → SH− :SH− + H+ → H2S Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides, polythionates, sulfite, or sulfate. Metal sulfides react with halogens, forming sulfur and metal salts. :8 MgS + 8 I2 → S8 + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Iron Minerals
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron Age. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |