Tetrahedrane From Diazo on:
[Wikipedia]
[Google]
[Amazon]
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of

 The tetrahedrane motif occurs broadly in chemistry. White phosphorus (P4) and
The tetrahedrane motif occurs broadly in chemistry. White phosphorus (P4) and
derivatives
The derivative of a function is the rate of change of the function's output relative to its input value.
Derivative may also refer to:
In mathematics and economics
* Brzozowski derivative in the theory of formal languages
* Formal derivative, an ...
have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.
Organic tetrahedranes
In 1978, Günther Maier prepared tetra- ''tert''-butyl-tetrahedrane. These bulky substituents envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting inVan der Waals strain Van der Waals strain is strain resulting from Van der Waals repulsion when two substituents in a molecule approach each other with a distance less than the sum of their Van der Waals radii.
Van der Waals strain is also called Van der Waals repul ...
. Tetrahedrane is one of the possible platonic hydrocarbons
In organic chemistry, a Platonic hydrocarbon is a hydrocarbon (molecule) whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed.
...
and has the IUPAC name tricyclo .1.0.02,4utane.
Unsubstituted tetrahedrane () remains elusive, although it is predicted to be kinetically stable. One strategy that has been explored (but thus far failed) is reaction of propene with atomic carbon
Atomic carbon, systematically named carbon and λ0-methane, also called monocarbon, is a colourless gaseous inorganic chemical with the chemical formula C (also written . It is kinetically unstable at ambient temperature and pressure, being remo ...
. Locking away a tetrahedrane molecule inside a fullerene has only been attempted ''in silico
In biology and other experimental sciences, an ''in silico'' experiment is one performed on computer or via computer simulation. The phrase is pseudo-Latin for 'in silicon' (correct la, in silicio), referring to silicon in computer chips. It ...
''. Due to its bond strain and stoichiometry, tetranitrotetrahedrane has potential as a high-performance energetic material (explosive). Some properties have been calculated based on quantum chemical methods.
Tetra-''tert''-butyltetrahedrane
This compound was first synthesised starting from a cycloaddition of analkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
with t-Bu substituted maleic anhydride, followed by rearrangement with carbon dioxide expulsion to a cyclopentadienone and its bromination, followed by addition of the fourth t-Bu group. Photochemical cheletropic elimination of carbon monoxide of the cyclopentadienone gives the target. Heating tetra-''tert''-butyltetrahedrane gives tetra-''tert''-butyl cyclobutadiene. Though the synthesis appears short and simple, by Maier's own account, it took several years of careful observation and optimization to develop the correct conditions for the challenging reactions to take place. For instance, the synthesis of tetrakis(''t-''butyl)cyclopentadienone from the tris(''t''-butyl)bromocyclopentadienone (itself synthesized with much difficulty) required over 50 attempts before working conditions could be found. The synthesis was described as requiring "astonishing persistence and experimental skill" in one retrospective of the work. In a classic reference work on stereochemistry, the authors remark that "the relatively straightforward scheme shown ..conceals both the limited availability of the starting material and the enormous amount of work required in establishing the proper conditions for each step."
:
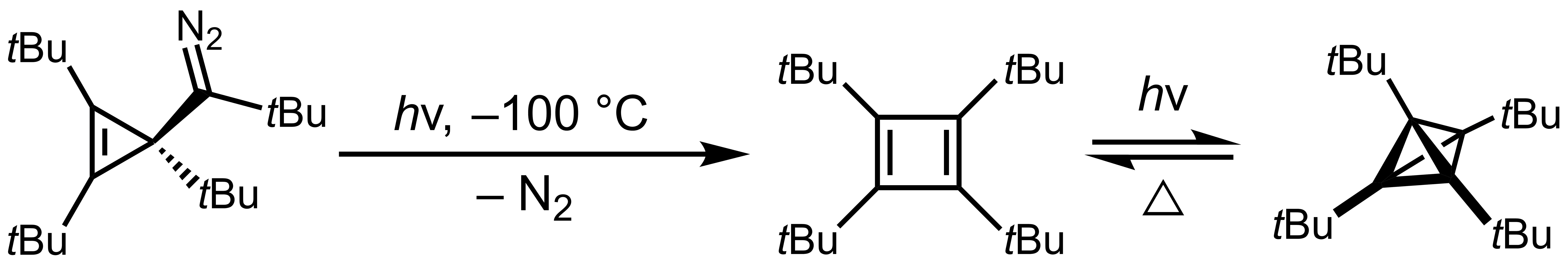
Eventually, a more scalable synthesis was conceived, in which the last step was the photolysis of a cyclopropenyl-substituted diazomethane, which affords the desired product through the intermediacy of tetrakis(''tert''-butyl)cyclobutadiene: This approach took advantage of the observation that the tetrahedrane and the cyclobutadiene could be interconverted (uv irradiation in the forward direction, heat in the reverse direction).
:
Tetrakis(trimethylsilyl)tetrahedrane
Tetrakis(trimethylsilyl)tetrahedrane can be prepared by treatment of the cyclobutadiene precursor withtris(pentafluorophenyl)borane
Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound . It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the c ...
and is far more stable than the ''tert''-butyl analogue. The silicon–carbon bond is longer than a carbon–carbon bond, and therefore the corset effect is reduced. Whereas the ''tert''-butyl tetrahedrane melts at 135 °C
The degree Celsius is the unit of temperature on the Celsius scale (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the Kelvin scale. The ...
concomitant with rearrangement to the cyclobutadiene, tetrakis(trimethylsilyl)tetrahedrane, which melts at 202 °C, is stable up to 300 °C, at which point it cracks to bis(trimethylsilyl)acetylene.
The tetrahedrane skeleton is made up of banana bond
In organic chemistry, a bent bond, also known as a banana bond, is a type of covalent chemical bond with a geometry somewhat reminiscent of a banana. The term itself is a general representation of electron density or configuration resembling a ...
s, and hence the carbon atoms are high in s-orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
character. From NMR, sp-hybridization
Hybridization (or hybridisation) may refer to:
*Hybridization (biology), the process of combining different varieties of organisms to create a hybrid
*Orbital hybridization, in chemistry, the mixing of atomic orbitals into new hybrid orbitals
*Nu ...
can be deduced, normally reserved for triple bonds. As a consequence the bond lengths are unusually short with 152 picometers.
Reaction with methyllithium with tetrakis(trimethylsilyl)tetrahedrane yields tetrahedranyllithium. Coupling reactions with this lithium compound gives extended structures.
A bis(tetrahedrane) has also been reported. The connecting bond is even shorter with 143.6 pm. An ordinary carbon–carbon bond has a length of 154 pm.
:
Tetrahedranes with non-carbon cores
In tetrasilatetrahedrane features a core of four silicon atoms. The standard silicon–silicon bond is much longer (235 pm) and the cage is again enveloped by a total of 16 trimethylsilyl groups, which confer stability. The silatetrahedrane can be reduced with potassium graphite to the tetrasilatetrahedranide potassium derivative. In this compound one of the silicon atoms of the cage has lost a silyl substituent and carries a negative charge. The potassium cation can be sequestered by a crown ether, and in the resulting complex potassium and the silyl anion are separated by a distance of 885 pm. One of the Si−–Si bonds is now 272 pm and its silicon atom has an inverted tetrahedral geometry. Furthermore, the four cage silicon atoms are equivalent on the NMR timescale due to migrations of the silyl substituents over the cage. : The dimerization reaction observed for the carbon tetrahedrane compound is also attempted for a tetrasilatetrahedrane. In this tetrahedrane the cage is protected by four so-called supersilyl groups in which a silicon atom has 3 ''tert''-butyl substituents. The dimer does not materialize but a reaction withiodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
in benzene followed by reaction with the tri-''tert''-butylsilaanion results in the formation of an eight-membered silicon cluster compound which can be described as a dumbbell (length 229 pm and with inversion of tetrahedral geometry) sandwiched between two almost-parallel rings.
:
In eight-membered clusters of in the same carbon group, tin and germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
the cluster atoms are located on the corners of a cube.
Inorganic and organometallic tetrahedranes
 The tetrahedrane motif occurs broadly in chemistry. White phosphorus (P4) and
The tetrahedrane motif occurs broadly in chemistry. White phosphorus (P4) and yellow arsenic
Arsenic in the solid state can be found as gray, black, or yellow allotropes. These various forms feature diverse structural motifs, with yellow arsenic enabling the widest range of reactivity. In particular, reaction of yellow arsenic with main gr ...
(As4) are examples. Several metal carbonyl clusters are referred to as tetrahedranes, e.g. tetrarhodium dodecacarbonyl.
Metallatetrahedranes with a single metal (or phosphorus atom) capping a cyclopropyl trianion also exist.
*Organometallics 2019, 38, 21, 4054–4059.
*Organometallics 1984, 3, 1574−1583.
*Organometallics 1986, 5, 25−33.
*J. Am. Chem. Soc. 1984, 106, 3356−3357.
*J. Chem. Soc., Chem. Commun. 1984, 485−486.
*Science Advances 25 Mar 2020: Vol. 6, no. 13, doi:10.1126/sciadv.aaz3168
See also
* Dodecahedrane * Prismane * PrismaneReferences
{{reflist, 30em Cycloalkanes Cluster chemistry Hypothetical chemical compounds Tricyclic compounds