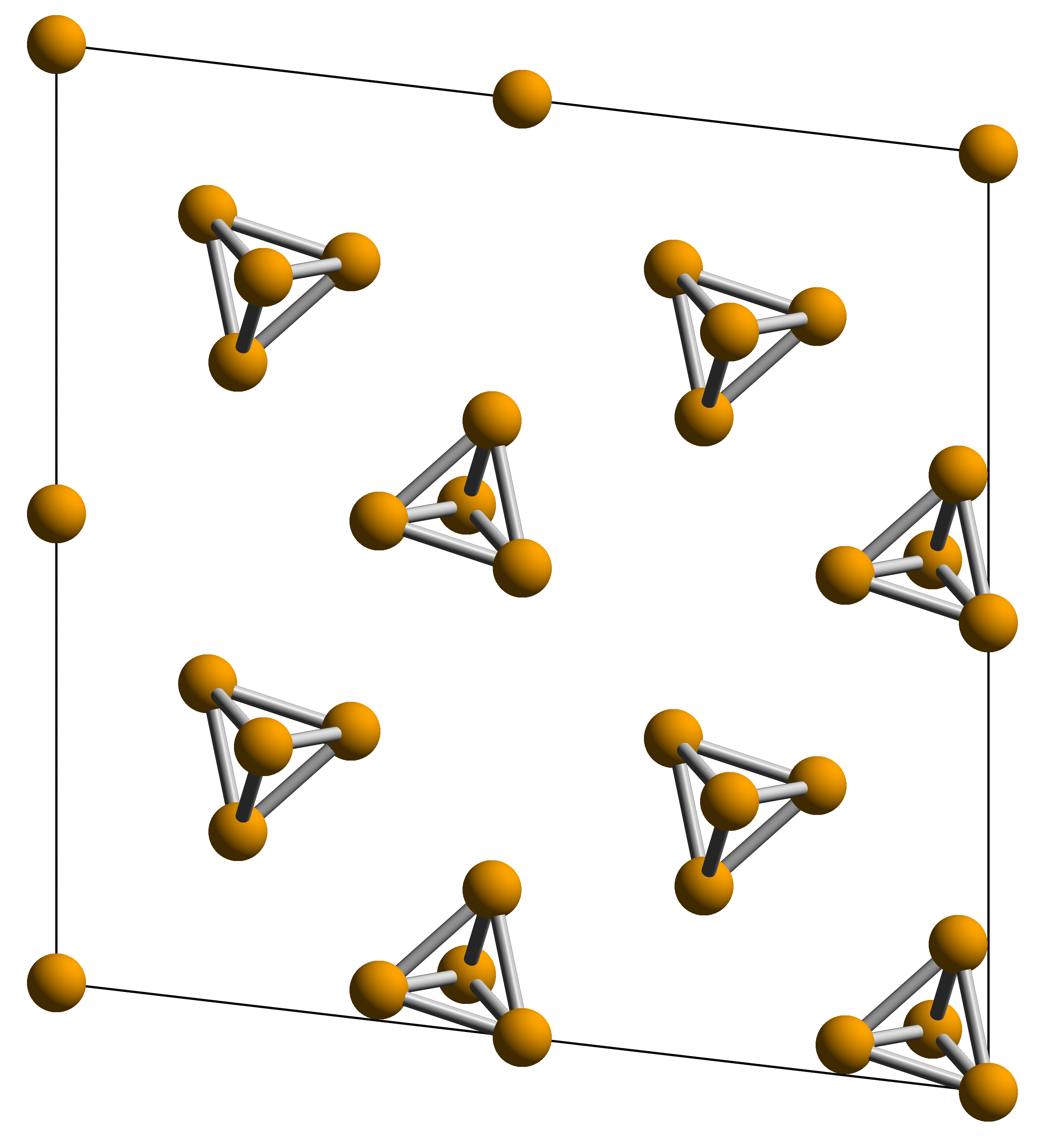|
White Phosphorus
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phosphorus is for this reason called yellow phosphorus. White phosphorus is the first allotrope of phosphorus, and in fact the first elementary substance to be discovered that was not known since ancient times. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air. It is toxic, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic odor, and samples are commonly coated with white " diphosphorus pentoxide", which consists of tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus. White phosphorus White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as molecules of four phosphorus atoms in a tetrahedral structure, joined by six phosphorus—phosphorus single bonds. The free P4 molecule in the gas phase has a P-P bond length of ''r''g = 2.1994(3) Å as was determined by gas electron diffraction. Despite the tetrahedral arrangement the P4 molecules have no significant ring strain and a vapor of P4 molecules is stable. This is due to the nature of bonding in the P4 tetrahedron which can be described by spherical aromaticity or cluster bonding, that is the electrons are highly delocalized. This has been illustrated by calculations of the magnetically induced currents, which sum up to 29 nA/T, much more than in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Single Bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of the two electrons involved is no longer in the sole possession of the Atomic orbital, orbital in which it originated. Rather, both of the two electrons spend time in either of the orbitals which overlap in the bonding process. As a Lewis structure, a single bond is denoted as AːA or A-A, for which A represents an element. In the first rendition, each dot represents a shared electron, and in the second rendition, the bar represents both of the electrons shared in the single bond. A covalent bond can also be a double bond or a triple bond. A single bond is weaker than either a double bond or a triple bond. This difference in strength can be explained by examining the component bonds of which each of these types of covalent bonds consists (Moo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxyacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid. Description Under Lavoisier's original theory, all acids contained oxygen, which was named from . It was later discovered that some acids, notably hydrochloric acid, did not contain oxygen and so acids were divided into oxo-acids and these new hydroacids. All oxyacids have the acidic hydrogen bound to an oxygen atom, so bond strength (length) is not a factor, as it is with binary nonmetal hydrides. Rather, the electronegativity of the central atom and the number of oxygen atoms determine oxyacid acidity. For oxyacids with the same central atom, acid strength increases with the number of oxygen atoms attached to it. With the same number of oxygen atoms attached to it, acid strength increases with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine () is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavoisi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called ''comproportionation'', also known as ''symproportionation''. More generally, the term can be applied to any desymmetrizing reaction where two molecules of one type react to give one each of two different types: : This expanded definition is not limited to redox reactions, but also includes some molecular autoionization reactions, such as the self-ionization of water. In contrast, some authors use the term ''redistribution'' to refer to reactions of this type (in either direction) when only ligand exchange but no redox is involved and distinguish such processes from disproportionation and comproportionati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chem)
In chemistry, there are three definitions in common use of the word "base": '' Arrhenius bases'', '' Brønsted bases'', and ''Lewis base A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...s''. All definitions agree that bases are substances that react with acids, as originally proposed by Guillaume-François Rouelle, G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form hydroxide ions OH−. These ions can react with Hydron (chemistry), hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Calcium hydroxide, Ca(OH)2. Such aqueous hydroxide solutions were also described by c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Enthalpy Of Formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, with all substances in their standard states. The standard pressure value is recommended by IUPAC, although prior to 1982 the value 1.00 atm (101.325 kPa) was used. There is no standard temperature. Its symbol is Δf''H''⦵. The superscript Plimsoll on this symbol indicates that the process has occurred under standard conditions at the specified temperature (usually 25 °C or 298.15 K). Standard states are defined for various types of substances. For a gas, it is the hypothetical state the gas would assume if it obeyed the ideal gas equation at a pressure of 1 bar. For a gaseous or solid solute present in a diluted ideal solution, the standard state is the hypothetical state of concentration of the solute of exactly one m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Electron Diffraction
Gas electron diffraction (GED) is one of the applications of electron diffraction techniques. The target of this method is the determination of the structure of gaseous molecules, i.e., the geometrical arrangement of the atoms from which a molecule is built up. GED is one of two experimental methods (besides microwave spectroscopy) to determine the structure of free molecules, undistorted by intermolecular forces, which are omnipresent in the solid and liquid state. The determination of accurate molecular structures by GED studies is fundamental for an understanding of structural chemistry. Introduction Diffraction occurs because the wavelength of electrons accelerated by a potential of a few thousand volts is of the same order of magnitude as internuclear distances in molecules. The principle is the same as that of other electron diffraction methods such as LEED and RHEED, but the obtainable diffraction pattern is considerably weaker than those of LEED and RHEED because the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°. Regular hexagon A regular hexagon is defined as a hexagon that is both equilateral and equiangular. In other words, a hexagon is said to be regular if the edges are all equal in length, and each of its internal angle is equal to 120°. The Schläfli symbol denotes this polygon as \ . However, the regular hexagon can also be considered as the cutting off the vertices of an equilateral triangle, which can also be denoted as \mathrm\ . A regular hexagon is bicentric, meaning that it is both cyclic (has a circumscribed circle) and tangential (has an inscribed circle). The common length of the sides equals the radius of the circumscribed circle or circumcircle, which equals \tfrac times the apothem (radius of the inscribed circle). Measurement The longest diagonal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Conditions
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used standards are those of the International Union of Pure and Applied Chemistry (IUPAC) and the National Institute of Standards and Technology (NIST), although these are not universally accepted. Other organizations have established a variety of other definitions. In industry and commerce, the standard conditions for temperature and pressure are often necessary for expressing the volumes of gases and liquids and related quantities such as the rate of volumetric flow (the volumes of gases vary significantly with temperature and pressure): standard cubic meters per second (Sm3/s), and normal cubic meters per second (Nm3/s). Many technical publications (books, journals, advertisements for equipment and machinery) simply state "standard cond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metastability
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy. A ball resting in a hollow on a slope is a simple example of metastability. If the ball is only slightly pushed, it will settle back into its hollow, but a stronger push may start the ball rolling down the slope. Bowling pins show similar metastability by either merely wobbling for a moment or tipping over completely. A common example of metastability in science is isomerisation. Higher energy isomers are long lived because they are prevented from rearranging to their preferred ground state by (possibly large) barriers in the potential energy. During a metastable state of finite lifetime, all state-describing parameters reach and hold stationary values. In isolation: *the state of least energy is the only one the system will inhabit for an indefinite length of time, until more external energy is added to the system (unique "absolu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Body-centered Cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal structure#Unit cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc) Note: the term fcc is often used in synonym for the Close-packing of equal spheres, ''cubic close-packed'' or ccp structure occurring in metals. However, fcc stands for a face-centered cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed. Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive cell, primitiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |