Allotropes of phosphorus on:
[Wikipedia]
[Google]
[Amazon]
 Elemental
Elemental 
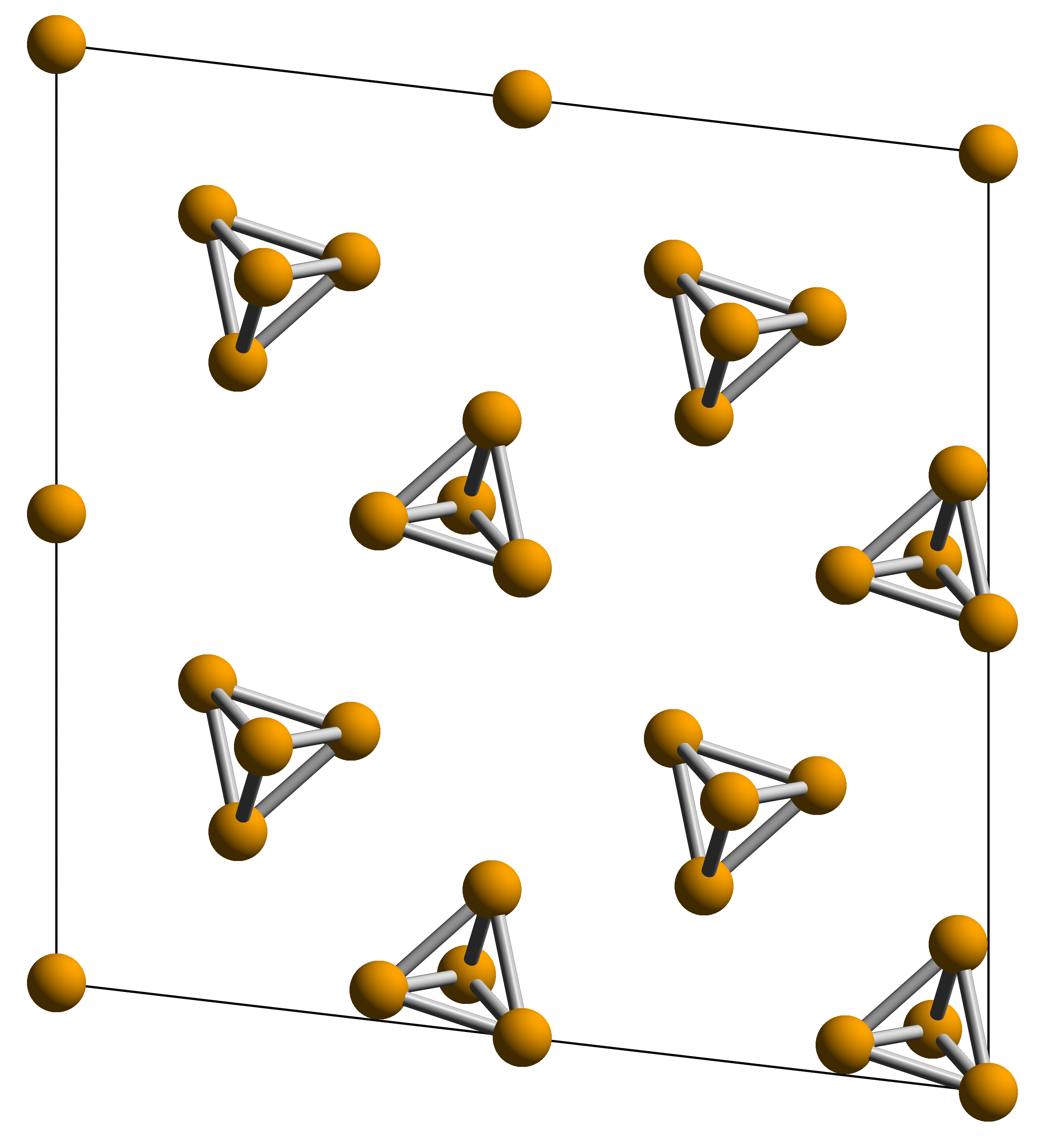
 White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as
White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as
 :
White phosphorus has an appreciable vapour pressure at ordinary temperatures. The vapour density indicates that the vapour is composed of molecules up to about 800 °C. Above that temperature, dissociation into molecules occurs.
It ignites spontaneously in air at about , and at much lower temperatures if finely divided (due to melting-point depression). Phosphorus reacts with oxygen, usually forming ''two'' oxides depending on the amount available oxygen: (
:
White phosphorus has an appreciable vapour pressure at ordinary temperatures. The vapour density indicates that the vapour is composed of molecules up to about 800 °C. Above that temperature, dissociation into molecules occurs.
It ignites spontaneously in air at about , and at much lower temperatures if finely divided (due to melting-point depression). Phosphorus reacts with oxygen, usually forming ''two'' oxides depending on the amount available oxygen: (

 Red phosphorus may be formed by heating
Red phosphorus may be formed by heating


 Monoclinic phosphorus, or violet phosphorus, is also known as Hittorf's metallic phosphorus. In 1865,
Monoclinic phosphorus, or violet phosphorus, is also known as Hittorf's metallic phosphorus. In 1865,
CSD-1935087
. The optical band gap of the violet phosphorus was measured by diffuse reflectance spectroscopy to be around 1.7 eV. The thermal decomposition temperature was 52 °C higher than its black phosphorus counterpart. The violet phosphorene was easily obtained from both mechanical and solution exfoliation.

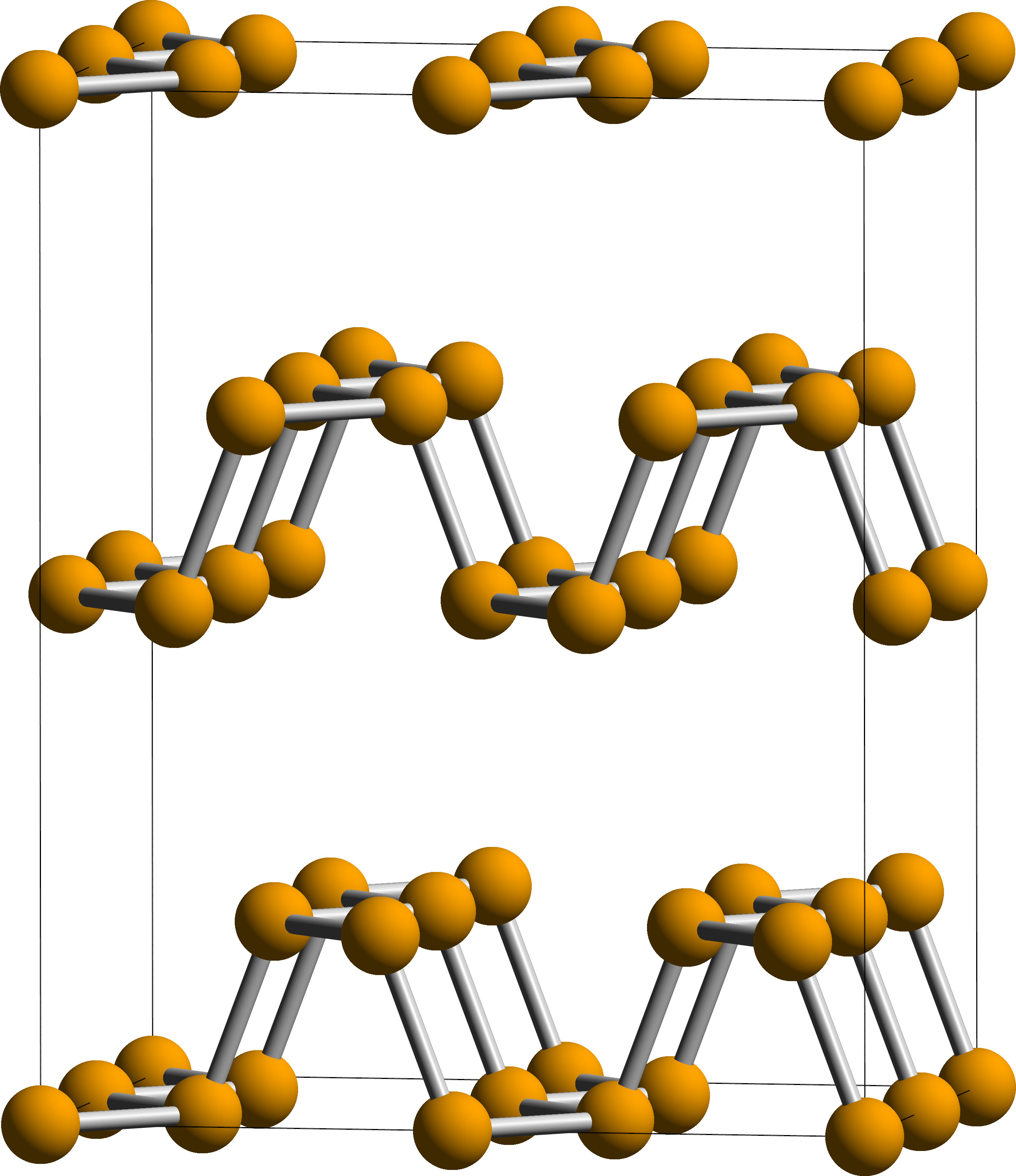
 Black phosphorus is the thermodynamically stable form of phosphorus at
Black phosphorus is the thermodynamically stable form of phosphorus at

 The diphosphorus allotrope () can normally be obtained only under extreme conditions (for example, from at 1100 kelvin). In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use of
The diphosphorus allotrope () can normally be obtained only under extreme conditions (for example, from at 1100 kelvin). In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use of
White Phophorus
at '' The Periodic Table of Videos'' (University of Nottingham)
More about White Phosphorus (and phosphorus pentoxide)
at '' The Periodic Table of Videos'' (University of Nottingham)
The Chemistry of Phosphorus
at Chemistry LibreTexts.
 Elemental
Elemental phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.White phosphorus
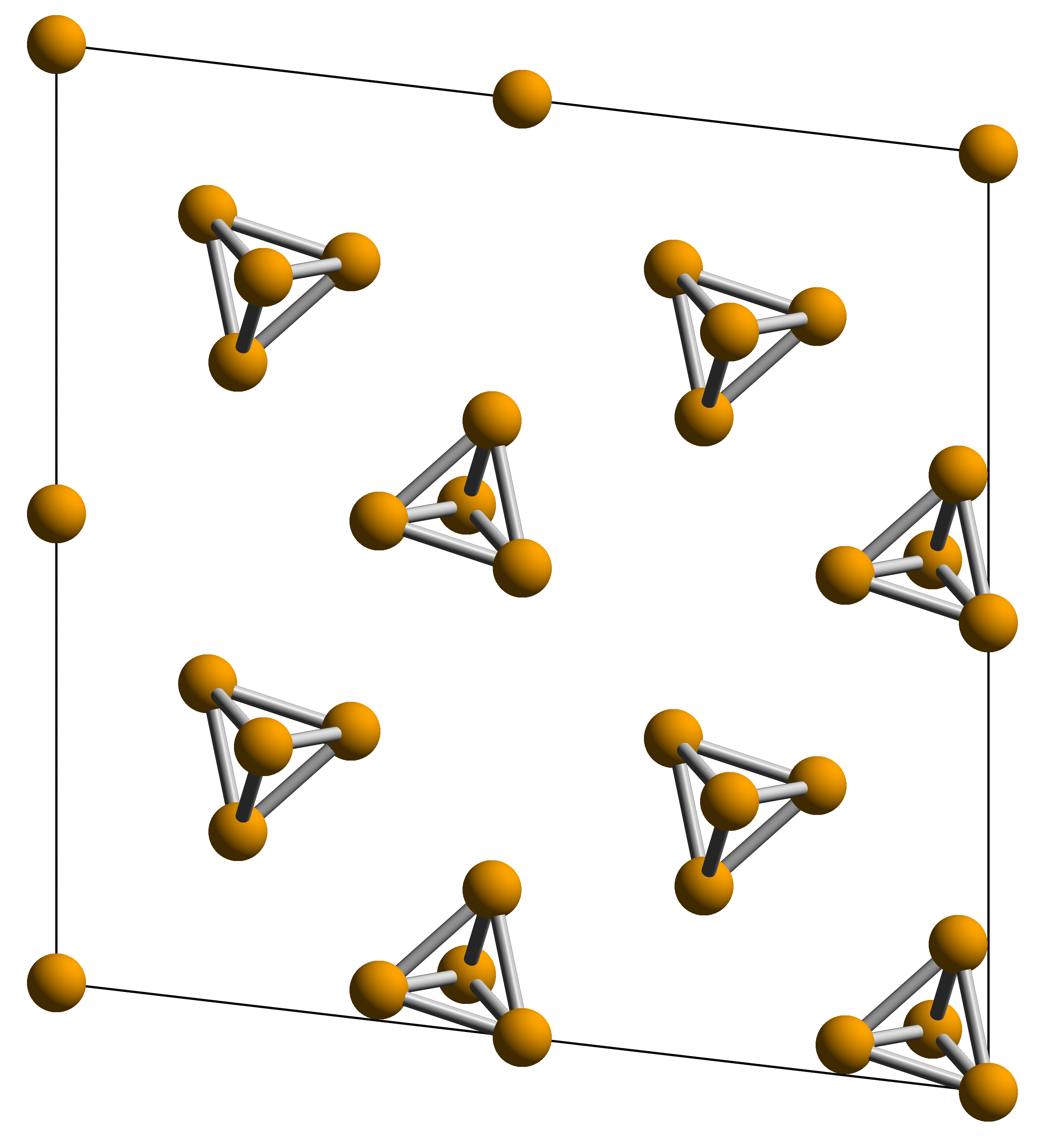
molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioc ...
made up of four atoms
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas ...
in a tetrahedral structure. The tetrahedral arrangement results in ring strain and instability. The molecule is described as consisting of six single P–P bonds. Two crystalline forms are known. The α form is defined as the standard state of the element, but is actually metastable
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball i ...
under standard conditions. It has a body-centered cubic crystal structure, and transforms reversibly into the β form at 195.2 K. The β form is believed to have a hexagonal crystal structure.
White phosphorus is a translucent waxy solid that quickly becomes yellow when exposed to light. For this reason it is also called yellow phosphorus. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
(self-igniting) upon contact with air. It is toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic smell, and samples are commonly coated with white "diphosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4 O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrat ...
", which consists of tetrahedral with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can be stored under water. Indeed, white phosphorus is safe from self-igniting only when it is submerged in water; due to this, unreacted white phosphorus can prove hazardous to beachcombers
''The Beachcombers'' is a Canadian comedy-drama television series that ran on CBC Television from October 1, 1972, to December 12, 1990. With over 350 episodes, it is one of the longest-running dramatic series ever made for English-language Canad ...
who may collect washed-up samples while unaware of their true nature. is soluble in benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
, oils
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) & lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturat ...
, carbon disulfide, and disulfur dichloride.
Production and applications
The white allotrope can be produced using several methods. In the industrial process, phosphate rock is heated in an electric or fuel-fired furnace in the presence ofcarbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes ...
and silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is o ...
.Threlfall, R.E., (1951). ''100 years of Phosphorus Making: 1851–1951''. Oldbury: Albright and Wilson Ltd Elemental phosphorus is then liberated as a vapour and can be collected under phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solutio ...
. An idealized equation for this carbothermal reaction is shown for calcium phosphate
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are wh ...
(although phosphate rock contains substantial amounts of fluoroapatite
Fluorapatite, often with the alternate spelling of fluoroapatite, is a phosphate mineral with the formula Ca5(PO4)3F (calcium fluorophosphate). Fluorapatite is a hard crystalline solid. Although samples can have various color (green, brown, bl ...
):
 :
White phosphorus has an appreciable vapour pressure at ordinary temperatures. The vapour density indicates that the vapour is composed of molecules up to about 800 °C. Above that temperature, dissociation into molecules occurs.
It ignites spontaneously in air at about , and at much lower temperatures if finely divided (due to melting-point depression). Phosphorus reacts with oxygen, usually forming ''two'' oxides depending on the amount available oxygen: (
:
White phosphorus has an appreciable vapour pressure at ordinary temperatures. The vapour density indicates that the vapour is composed of molecules up to about 800 °C. Above that temperature, dissociation into molecules occurs.
It ignites spontaneously in air at about , and at much lower temperatures if finely divided (due to melting-point depression). Phosphorus reacts with oxygen, usually forming ''two'' oxides depending on the amount available oxygen: (phosphorus trioxide
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexaoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, a ...
) when reacted with a limited supply of oxygen, and when reacted with excess oxygen. On rare occasions, , , and are also formed, but in small amounts. This combustion gives phosphorus(V) oxide:
:
Because of this property, white phosphorus is used as a weapon.
Non-existence of cubic-
Although white phosphorus converts to the thermodynamically more stable red allotrope, the formation of the cubic- molecule is not observed in the condensed phase. Analogs of this hypothetical molecule have been prepared from phosphaalkynes. White phosphorus in the gaseous state and as waxy solid consists of reactive molecules.Red phosphorus

 Red phosphorus may be formed by heating
Red phosphorus may be formed by heating white phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
White phosphorus
Whi ...
to in the absence of air or by exposing white phosphorus to sunlight. Red phosphorus exists as an amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek language ...
network. Upon further heating, the amorphous red phosphorus crystallizes. Red phosphorus does not ignite in air at temperatures below , whereas pieces of white phosphorus ignite at about . Ignition is spontaneous at room temperature with finely divided material as the high surface area allows the surface oxidation to rapidly heat the sample to the ignition temperature.
Under standard conditions it is more stable than white phosphorus, but less stable than the thermodynamically stable black phosphorus. The standard enthalpy of formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, wi ...
of red phosphorus is −17.6 kJ/mol. Red phosphorus is kinetically most stable.
It was first presented by Anton von Schrötter before the Vienna Academy of Sciences on December 9, 1847, although others had doubtless had this substance in their hands before, such as Berzelius.
Applications
Red phosphorus can be used as a very effectiveflame retardant
The term flame retardants subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source a ...
, especially in thermoplastic
A thermoplastic, or thermosoft plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains associat ...
s (e.g. polyamide) and thermosets
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer ( resin). Curing is induced by heat or suitable radiation an ...
(e.g. epoxy resins or polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethan ...
s). The flame retarding effect is based on the formation of polyphosphoric acid. Together with the organic polymer material, these acids create a char that prevents the propagation of the flames. The safety risks associated with phosphine
Phosphine ( IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotti ...
generation and friction sensitivity
Friction sensitivity is an approximation of the amount of friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. There are several types of friction:
* ...
of red phosphorus can be effectively minimized by stabilization and micro-encapsulation. For easier handling, red phosphorus is often used in form of dispersions or masterbatches in various carrier systems. However, for electronic/electrical systems, red phosphorus flame retardant has been effectively banned by major OEMs due to its tendency to induce premature failures. One persistent problem is that red phosphorus in epoxy molding compounds induces elevated leakage current in semiconductor devices. Another problem was acceleration of hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
reactions in PBT insulating material.
Red phosphorus can also be used in the illicit production of methamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder and obesity. Meth ...
and Krokodil
''Krokodil'' ( rus, Крокодил, p=krəkɐˈdʲil, a= Ru-крокодил.ogg, ) was a satirical magazine published in the Soviet Union. It was founded in 1922 as the satirical supplement to the '' Workers' Gazette'' (called simply «При� ...
.
Red phosphorus can be used as an elemental photocatalyst for hydrogen formation from the water. They display a steady hydrogen evolution rates of 633 μmol/(h⋅g) by the formation of small-sized fibrous phosphorus.
Violet or Hittorf's phosphorus

 Monoclinic phosphorus, or violet phosphorus, is also known as Hittorf's metallic phosphorus. In 1865,
Monoclinic phosphorus, or violet phosphorus, is also known as Hittorf's metallic phosphorus. In 1865, Johann Wilhelm Hittorf
Johann Wilhelm Hittorf (27 March 1824 – 28 November 1914) was a German physicist who was born in Bonn and died in Münster, Germany.
Hittorf was the first to compute the electricity-carrying capacity of charged atoms and molecules (ions), an ...
heated red phosphorus in a sealed tube at 530 °C. The upper part of the tube was kept at 444 °C. Brilliant opaque monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic ...
, or rhombohedral, crystals sublimed as a result. Violet phosphorus can also be prepared by dissolving white phosphorus in molten lead
Lead is a chemical element with the Symbol (chemistry), symbol Pb (from the Latin ) and atomic number 82. It is a heavy metals, heavy metal that is density, denser than most common materials. Lead is Mohs scale of mineral hardness#Intermediate ...
in a sealed tube at 500 °C for 18 hours. Upon slow cooling, Hittorf's allotrope crystallises out. The crystals can be revealed by dissolving the lead in dilute nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
followed by boiling in concentrated hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
. In addition, a fibrous form exists with similar phosphorus cages. The lattice structure of violet phosphorus was presented by Thurn and Krebs in 1969. Imaginary frequencies, indicating the irrationalities or instabilities of the structure, were obtained for the reported violet structure from 1969. The single crystal of violet phosphorus was also produced. The lattice structure of violet phosphorus has been obtained by single‐crystal ''x''‐ray diffraction to be monoclinic with space group of ''P''2/''n'' (13) (''a'' = 9.210, ''b'' = 9.128, ''c'' = 21.893 Å, ''β'' = 97.776°CSD-1935087
. The optical band gap of the violet phosphorus was measured by diffuse reflectance spectroscopy to be around 1.7 eV. The thermal decomposition temperature was 52 °C higher than its black phosphorus counterpart. The violet phosphorene was easily obtained from both mechanical and solution exfoliation.
Reactions of violet phosphorus
It does not ignite in air until heated to 300 °C and is insoluble in all solvents. It is not attacked byalkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of ...
and only slowly reacts with halogens
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group ...
. It can be oxidised by nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
to phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solutio ...
.
If it is heated in an atmosphere of inert gas, for example nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
or carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, it sublimes
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point i ...
and the vapour condenses as white phosphorus. If it is heated in a vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or " void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often di ...
and the vapour condensed rapidly, violet phosphorus is obtained. It would appear that violet phosphorus is a polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
of high relative molecular mass, which on heating breaks down into molecules. On cooling, these would normally dimerize to give molecules (i.e. white phosphorus) but, in a vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or " void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often di ...
, they link up again to form the polymeric violet allotrope.
Black phosphorus


room temperature and pressure
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
, with a heat of formation of −39.3 kJ/mol (relative to white phosphorus which is defined as the standard state). It was first synthesized by heating white phosphorus under high pressures (12,000 atmospheres) in 1914. As a 2D material, in appearance, properties, and structure, black phosphorus is very much like graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
with both being black and flaky, a conductor of electricity, and having puckered sheets of linked atoms.
Black phosphorus has an orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with ...
pleated honeycomb structure and is the least reactive allotrope, a result of its lattice of interlinked six-membered rings where each atom is bonded to three other atoms. In this structure, each phosphorus atom has five outer shell electrons. Black and red phosphorus can also take a cubic
Cubic may refer to:
Science and mathematics
* Cube (algebra), "cubic" measurement
* Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex
** Cubic crystal system, a crystal system w ...
crystal lattice structure. The first high-pressure synthesis of black phosphorus crystals was made by the Nobel prize winner Percy Williams Bridgman
Percy Williams Bridgman (April 21, 1882 – August 20, 1961) was an American physicist who received the 1946 Nobel Prize in Physics for his work on the physics of high pressures. He also wrote extensively on the scientific method and on other as ...
in 1914. Metal salts catalyze the synthesis of black phosphorus.
Phosphorene
The similarities to graphite also include the possibility of scotch-tape delamination (exfoliation), resulting in phosphorene, agraphene
Graphene () is an allotrope of carbon consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice nanostructure.
-like 2D material with excellent charge transport properties, thermal transport properties and optical properties. Distinguishing features of scientific interest include a thickness dependent band-gap, which is not found in graphene. This, combined with a high on/off ratio of ~105 makes phosphorene a promising candidate for field-effect transistors (FETs). The tunable bandgap also suggests promising applications in mid-infrared photodetectors and LEDs. Exfoliated black phosphorus sublimes at 400 °C in vacuum. It gradually oxidizes when exposed to water in the presence of oxygen, which is a concern when contemplating it as a material for the manufacture of transistors, for example. Exfoliated black phosphorus is an emerging anode material in the battery community, showing high stability and lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
storage.
Ring-shaped phosphorus
Ring-shaped phosphorus was theoretically predicted in 2007. The ring-shaped phosphorus was self-assembled inside evacuated multi-walled carbon nanotubes with inner diameters of 5–8 nm using a vapor encapsulation method. A ring with a diameter of 5.30 nm, consisting of 23 and 23 units with a total of 230 P atoms, was observed inside a multi-walled carbon nanotube with an inner diameter of 5.90 nm in atomic scale. The distance between neighboring rings is 6.4 Å. The ring shaped molecule is not stable in isolation.Blue phosphorus
Single-layer blue phosphorus was first produced in 2016 by the method of molecular beam epitaxy from black phosphorus as precursor.Diphosphorus

 The diphosphorus allotrope () can normally be obtained only under extreme conditions (for example, from at 1100 kelvin). In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use of
The diphosphorus allotrope () can normally be obtained only under extreme conditions (for example, from at 1100 kelvin). In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use of transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that c ...
complexes (for example, tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
and niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it ha ...
).
Diphosphorus is the gaseous form of phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
, and the thermodynamically stable form between 1200 °C and 2000 °C. The dissociation of tetraphosphorus () begins at lower temperature: the percentage of at 800 °C is ≈ 1%. At temperatures above about 2000 °C, the diphosphorus molecule begins to dissociate into atomic phosphorus.
Phosphorus nanorods
nanorod polymers were isolated from CuI-P complexes using low temperature treatment. Red/brown phosphorus was shown to be stable in air for several weeks and have properties distinct from those of red phosphorus. Electron microscopy showed that red/brown phosphorus forms long, parallel nanorods with a diameter between 3.4 Å and 4.7 Å.Properties
See also
* Phossy jawReferences
{{reflist, 30emExternal links
;White phosphorusWhite Phophorus
at '' The Periodic Table of Videos'' (University of Nottingham)
More about White Phosphorus (and phosphorus pentoxide)
at '' The Periodic Table of Videos'' (University of Nottingham)
The Chemistry of Phosphorus
at Chemistry LibreTexts.
Phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
Phosphorus