|
Fluoroapatite
Fluorapatite, often with the alternate spelling of fluoroapatite, is a phosphate mineral with the formula Ca5(PO4)3F (calcium fluorophosphate). Fluorapatite is a hard crystalline solid. Although samples can have various color (green, brown, blue, yellow, violet, or colorless), the pure mineral is colorless, as expected for a material lacking transition metals. Along with hydroxylapatite, it can be a component of tooth enamel, but for industrial use both minerals are mined in the form of phosphate rock, whose usual mineral composition is primarily fluorapatite but often with significant amounts of the other. Fluorapatite crystallizes in a hexagonal crystal system. It is often combined as a solid solution with hydroxylapatite (Ca5(PO4)3OH or Ca10(PO4)6(OH)2) in biological matrices. Chlorapatite (Ca5(PO4)3Cl) is another related structure. Industrially, the mineral is an important source of both phosphoric and hydrofluoric acids. Fluorapatite as a mineral is the most common p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10( PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2. The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means ''to deceive''. Geology Apatite is very common as an accessory mineral in igneous and metamorphic rocks, where it is the most common phosphate mineral. However, occu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a Solution (chemistry), solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly Corrosive substance, corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material polytetrafluoroethylene, PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to Etching (microfabrication), etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Polytetrafluoroethylene, Teflon, fluoropolymers, fluorocarbons, and refrigeration, refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorapatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10( PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2. The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means ''to deceive''. Geology Apatite is very common as an accessory mineral in igneous and metamorphic rocks, where it is the most common phosphate mineral. However, occurre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Fluoridation
Water fluoridation is the controlled adjustment of fluoride to a public water supply solely to reduce tooth decay. Fluoridated water contains fluoride at a level that is effective for preventing cavities; this can occur naturally or by adding fluoride. Fluoridated water operates on tooth surfaces: in the mouth, it creates low levels of fluoride in saliva, which reduces the rate at which tooth enamel demineralizes and increases the rate at which it remineralizes in the early stages of cavities. Typically a fluoridated compound is added to drinking water, a process that in the U.S. costs an average of about $ per person-year. Defluoridation is needed when the naturally occurring fluoride level exceeds recommended limits. In 2011, the World Health Organization suggested a level of fluoride from 0.5 to 1.5 mg/L (milligrams per litre), depending on climate, local environment, and other sources of fluoride. Bottled water typically has unknown fluoride levels. Tooth decay rem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laterite
Laterite is both a soil and a rock type rich in iron and aluminium and is commonly considered to have formed in hot and wet tropical areas. Nearly all laterites are of rusty-red coloration, because of high iron oxide content. They develop by intensive and prolonged weathering of the underlying parent rock, usually when there are conditions of high temperatures and heavy rainfall with alternate wet and dry periods. Tropical weathering (''laterization'') is a prolonged process of chemical weathering which produces a wide variety in the thickness, grade, chemistry and ore mineralogy of the resulting soils. The majority of the land area containing laterites is between the tropics of Cancer and Capricorn. Laterite has commonly been referred to as a soil type as well as being a rock type. This and further variation in the modes of conceptualizing about laterite (e.g. also as a complete weathering profile or theory about weathering) has led to calls for the term to be abandoned alto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shark
Sharks are a group of elasmobranch fish characterized by a cartilaginous skeleton, five to seven gill slits on the sides of the head, and pectoral fins that are not fused to the head. Modern sharks are classified within the clade Selachimorpha (or Selachii) and are the sister group to the rays. However, the term "shark" has also been used to refer to all extinct members of Chondrichthyes with a shark-like morphology, such as hybodonts and xenacanths. The oldest modern sharks are known from the Early Jurassic. They range in size from the small dwarf lanternshark (''Etmopterus perryi''), a deep sea species that is only in length, to the whale shark (''Rhincodon typus''), the largest fish in the world, which reaches approximately in length. Sharks are found in all seas and are common to depths up to . They generally do not live in freshwater, although there are a few known exceptions, such as the bull shark and the river shark, which can be found in both seawater and fresh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
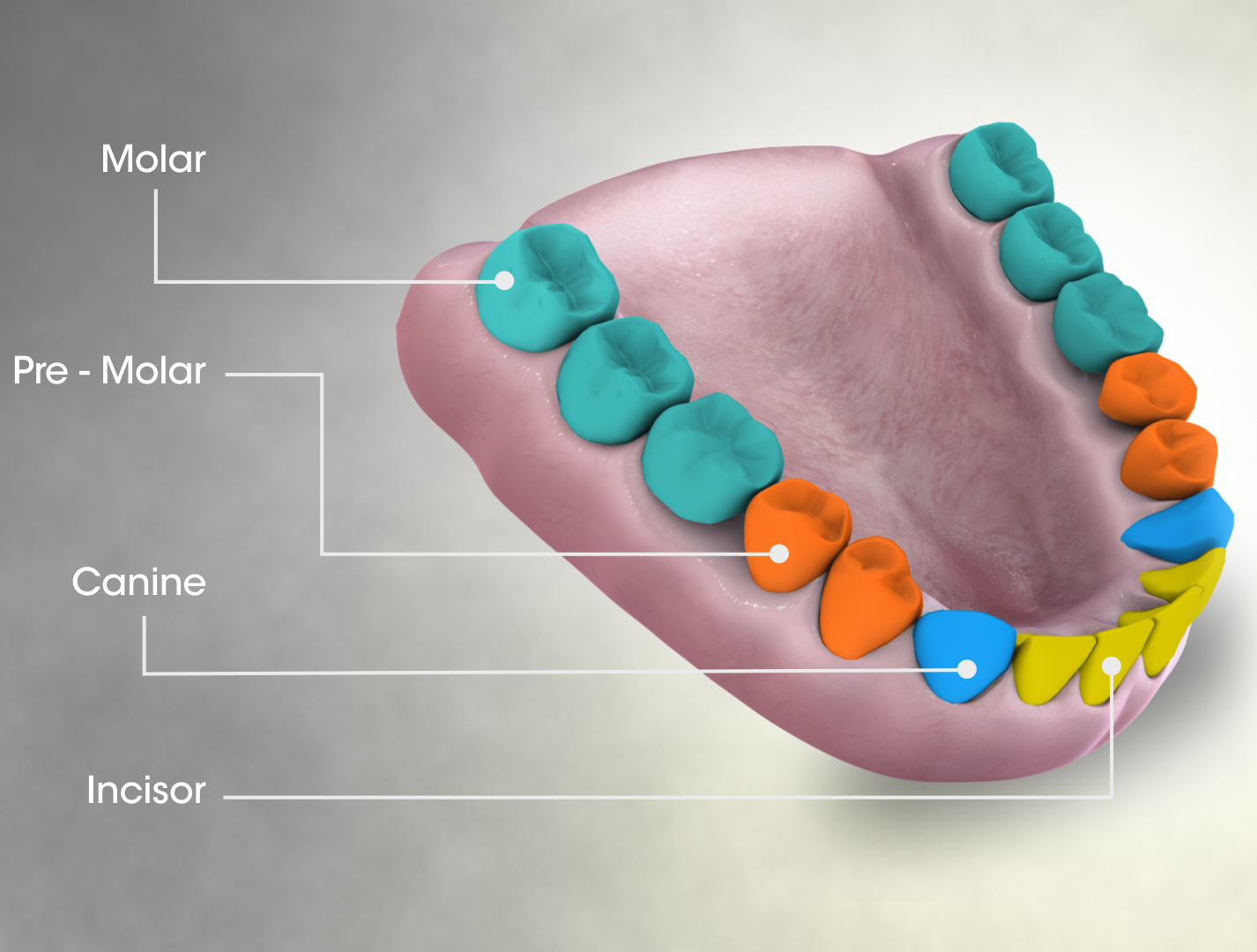
Human Teeth
The human teeth function to mechanically break down items of food by cutting and crushing them in preparation for swallowing and digesting. As such, they are considered part of the human digestive system. Humans have four types of teeth: incisors, canines, premolars, and molars, which each have a specific function. The incisors cut the food, the canines tear the food and the molars and premolars crush the food. The roots of teeth are embedded in the maxilla (upper jaw) or the mandible (lower jaw) and are covered by gums. Teeth are made of multiple tissues of varying density and hardness. Humans, like most other mammals, are diphyodont, meaning that they develop two sets of teeth. The first set, deciduous teeth, also called "primary teeth", "baby teeth", or "milk teeth", normally eventually contains 20 teeth. Primary teeth typically start to appear (" erupt") around six months of age and this may be distracting and/or painful for the infant. However, some babies are born with o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The nom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate Mineral
Phosphate minerals contain the tetrahedrally coordinated phosphate (PO43−) anion along sometimes with arsenate (AsO43−) and vanadate (VO43−) substitutions, and chloride (Cl−), fluoride (F−), and hydroxide (OH−) anions that also fit into the crystal structure. The phosphate class of minerals is a large and diverse group, however, only a few species are relatively common. Applications Phosphate rock has high concentration of phosphate minerals, most commonly of the apatite group. It is the major resource mined to produce phosphate fertilizers for the agriculture sector. Phosphate is also used in animal feed supplements, food preservatives, anti-corrosion agents, cosmetics, fungicides, ceramics, water treatment and metallurgy. The largest use of minerals mined for their phosphate content is the production of fertilizer. Phosphate minerals are often used for control of rust and prevention of corrosion on ferrous materials applied with electrochemical conversion co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorite
Phosphorite, phosphate rock or rock phosphate is a non-detrital sedimentary rock that contains high amounts of phosphate minerals. The phosphate content of phosphorite (or grade of phosphate rock) varies greatly, from 4% to 20% phosphorus pentoxide (P2O5). Marketed phosphate rock is enriched ("beneficiated") to at least 28%, often more than 30% P2O5. This occurs through washing, screening, de-liming, magnetic separation or flotation. By comparison, the average phosphorus content of sedimentary rocks is less than 0.2%.Blatt, Harvey and Robert J. Tracy, ''Petrology'', Freeman, 1996, 2nd ed. pp. 345–349 The phosphate is present as fluorapatite Ca5(PO4)3F typically in cryptocrystalline masses (grain sizes < 1 μm) referred to as -sedimentary apatite deposits of uncertain origin. It is also present as |
Dental Caries
Tooth decay, also known as cavities or caries, is the breakdown of teeth due to acids produced by bacteria. The cavities may be a number of different colors from yellow to black. Symptoms may include pain and difficulty with eating. Complications may include inflammation of the tissue around the tooth, tooth loss and infection or abscess formation. The cause of cavities is acid from bacteria dissolving the hard tissues of the teeth ( enamel, dentin and cementum). The acid is produced by the bacteria when they break down food debris or sugar on the tooth surface. Simple sugars in food are these bacteria's primary energy source and thus a diet high in simple sugar is a risk factor. If mineral breakdown is greater than build up from sources such as saliva, caries results. Risk factors include conditions that result in less saliva such as: diabetes mellitus, Sjögren syndrome and some medications. Medications that decrease saliva production include antihistamines and antide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptococcus Mutans
''Streptococcus mutans'' is a facultatively anaerobic, gram-positive coccus (round bacterium) commonly found in the human oral cavity and is a significant contributor to tooth decay. It is part of the " streptococci" (plural, non-italic lowercase), an informal general name for all species in the genus ''Streptococcus''. The microbe was first described by James Kilian Clarke in 1924. This bacterium, along with the closely related species ''Streptococcus sobrinus'', can cohabit the mouth: Both contribute to oral disease, and the expense of differentiating them in laboratory testing is often not clinically necessary. Therefore, for clinical purposes they are often considered together as a group, called the mutans streptococci (plural, non-italic due to its being an informal group name). This grouping of similar bacteria with similar tropism can also be seen in the viridans streptococci, another group of ''Streptococcus'' species. Ecology ''S. mutans'' is naturally present in the hu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


_0468.jpg)