|
Dodecahedrane
Dodecahedrane is a chemical compound, a hydrocarbon with formula , whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron. Each carbon is bound to three neighbouring carbon atoms and to a hydrogen atom. This compound is one of the three possible Platonic hydrocarbons, the other two being cubane and tetrahedrane. Dodecahedrane does not occur in nature and has no significant uses. It was synthesized by Leo Paquette in 1982, primarily for the "aesthetically pleasing symmetry of the dodecahedral framework". For many years, dodecahedrane was the simplest real carbon-based molecule with full icosahedral symmetry. Buckminsterfullerene (), discovered in 1985, also has the same symmetry, but has three times as many carbons and 50% more atoms overall. The synthesis of the C20 fullerene in 2000, from brominated dodecahedrane, may have demoted to second place. Structure The angle between the C-C bonds in each carbon atom is 108°, which is the angle between a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dodecahedrane
Dodecahedrane is a chemical compound, a hydrocarbon with formula , whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron. Each carbon is bound to three neighbouring carbon atoms and to a hydrogen atom. This compound is one of the three possible Platonic hydrocarbons, the other two being cubane and tetrahedrane. Dodecahedrane does not occur in nature and has no significant uses. It was synthesized by Leo Paquette in 1982, primarily for the "aesthetically pleasing symmetry of the dodecahedral framework". For many years, dodecahedrane was the simplest real carbon-based molecule with full icosahedral symmetry. Buckminsterfullerene (), discovered in 1985, also has the same symmetry, but has three times as many carbons and 50% more atoms overall. The synthesis of the C20 fullerene in 2000, from brominated dodecahedrane, may have demoted to second place. Structure The angle between the C-C bonds in each carbon atom is 108°, which is the angle between a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platonic Hydrocarbons
In organic chemistry, a Platonic hydrocarbon is a hydrocarbon (molecule) whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed. Not all Platonic solids have molecular hydrocarbon counterparts; those that do are the tetrahedron (tetrahedrane), the cube (cubane), and the dodecahedron (dodecahedrane). Tetrahedrane Tetrahedrane (C4H4) is a hypothetical compound. It has not yet been synthesized without substituents, but it is predicted to be kinetically stable in spite of its angle strain. Some stable derivatives, including tetra( ''tert''-butyl)tetrahedrane (a hydrocarbon) and tetra(trimethylsilyl)tetrahedrane, have been produced. Cubane Cubane (C8H8) has been synthesized. Although it has high angle strain, cubane is kinetically stable, due to a lack of readily available decomposition paths. Octahedrane Angle strain would make an octahedron highly unstable due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
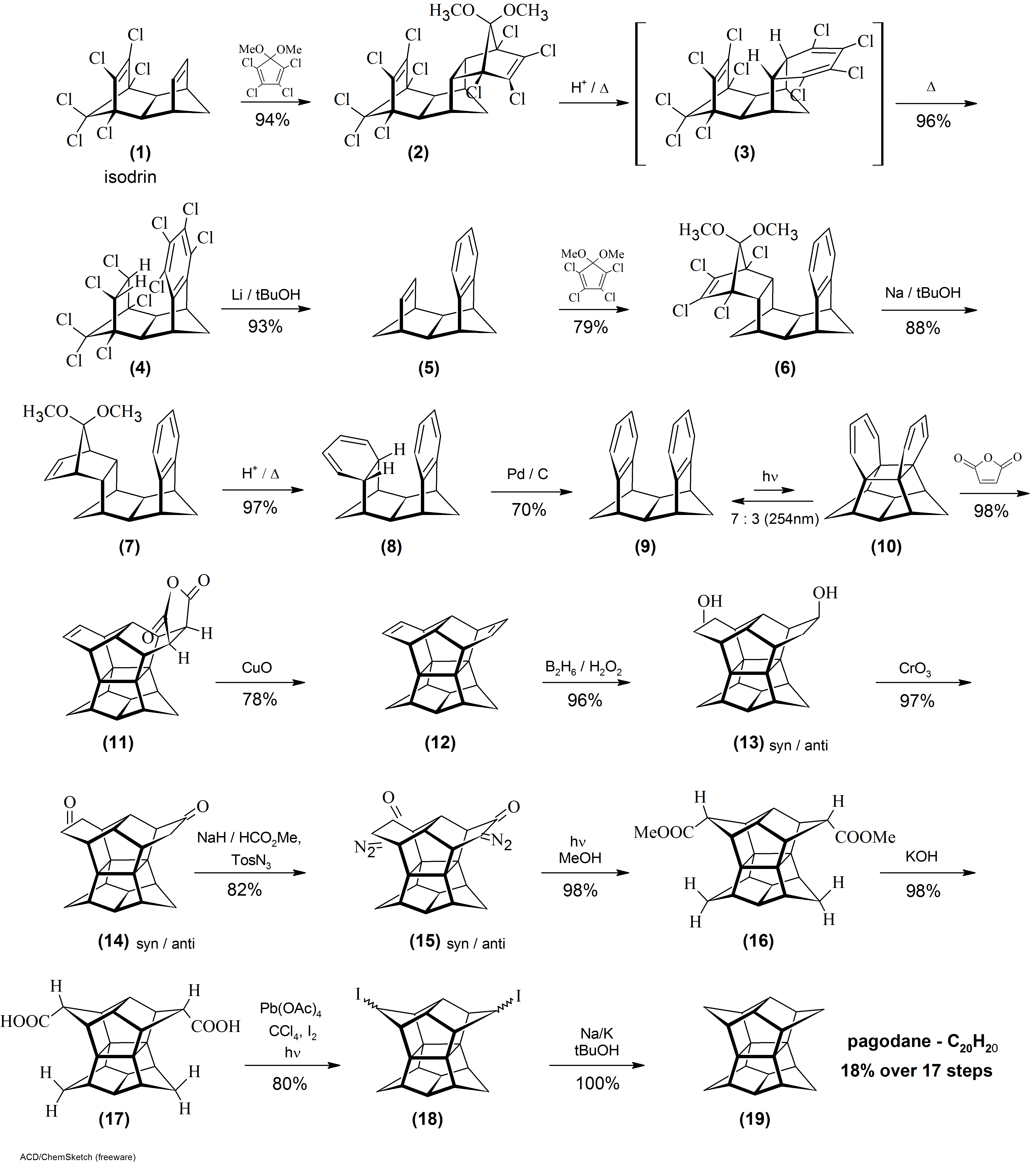
Pagodane
Pagodane is an organic compound with formula whose carbon skeleton was said to resemble a pagoda, hence the name. It is a polycyclic hydrocarbon whose molecule has the ''D''2''h'' point symmetry group. The compound is a highly crystalline solid that melts at 243 °C, is barely soluble in most organic solvents and moderately soluble in benzene and chloroform. It sublimes at low pressure. The name pagodane is used more generally for any member of a family of compounds whose molecular skeletons have the same 16-carbon central cage as the basic compound. Each member can be seen as the result of connecting eight atoms of this cage in pairs by four alkane chains. The general member is denoted 'm''.''n''.''p''.''q''agodane where ''m'', ''n'', ''p'' and ''q'' are the number of carbons of those four chains. The general formula is then where ''s''= ''m''+''n''+''p''+''q''. In particular, the basic compound has those carbons connected by four methylene bridges (''m''=''n''=''p''=''q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leo Paquette
Leo Armand Paquette ( – January 21, 2019) was an American organic chemist. Biography He was born on July 15, 1934 to parents Armand and Clarice with roots in Quebec (great-grandfather Edmund was born in Contrecoeur, Quebec) and he received his B.S. degree from Holy Cross College in 1956 and his Ph.D. in Organic Chemistry from the Massachusetts Institute of Technology in 1959 with Professor Norman Allan Nelson. After serving as a research associate at the Upjohn Company from 1959 to 1963, he joined the faculty of Ohio State University. He was promoted to full professor in 1969 and was named Distinguished University Professor in 1987. Paquette was elected a member of the National Academy of Sciences in 1984, and was the founding editor of the '' Electronic Encyclopedia of Reagents for Organic Synthesis (e-EROS). Paquette is perhaps best known for achieving the first total synthesis of the Platonic solid dodecahedrane. Scientific misconduct In 1993, an Ohio State University i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
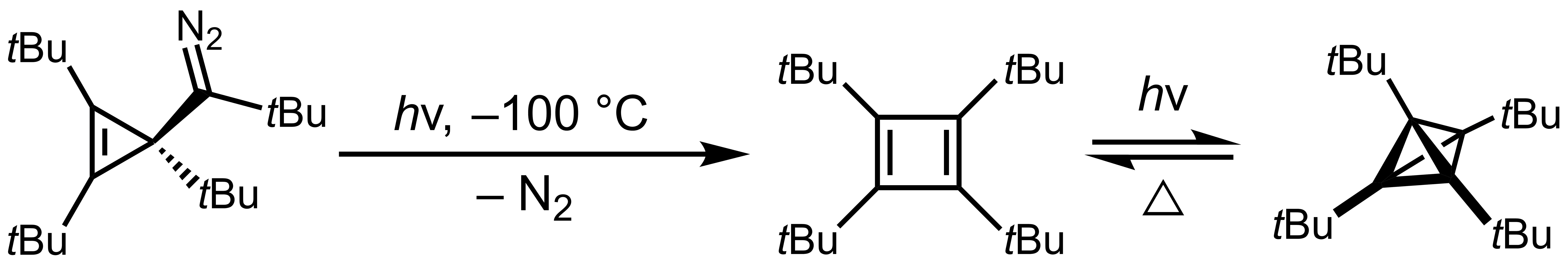
Tetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus. Organic tetrahedranes In 1978, Günther Maier prepared tetra- ''tert''-butyl-tetrahedrane. These bulky substituents envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting in Van der Waals strain. Tetrahedrane is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane () remains elusive, although it is predicted to be kinetically stable. One strategy that has been explored (but thus far failed) i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubane
Cubane () is a synthetic hydrocarbon compound that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry. Having high potential energy but kinetic stability makes cubane and its derivative compounds useful for controlled energy storage. For example, octanitrocubane and heptanitrocubane have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical properties, such as whether or not it has a dipole moment, as well as its allowed spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward-Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry. Another framework on a larger scale is the use of crystal systems to describe crystallographic symmetry in bulk materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus. Organic tetrahedranes In 1978, Günther Maier prepared tetra- ''tert''-butyl-tetrahedrane. These bulky substituents envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting in Van der Waals strain. Tetrahedrane is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane () remains elusive, although it is predicted to be kinetically stable. One strategy that has been explored (but thus far failed) i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubane
Cubane () is a synthetic hydrocarbon compound that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry. Having high potential energy but kinetic stability makes cubane and its derivative compounds useful for controlled energy storage. For example, octanitrocubane and heptanitrocubane have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regular Tetrahedron
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ordinary convex polyhedra and the only one that has fewer than 5 faces. The tetrahedron is the three-dimensional case of the more general concept of a Euclidean simplex, and may thus also be called a 3-simplex. The tetrahedron is one kind of pyramid, which is a polyhedron with a flat polygon base and triangular faces connecting the base to a common point. In the case of a tetrahedron the base is a triangle (any of the four faces can be considered the base), so a tetrahedron is also known as a "triangular pyramid". Like all convex polyhedra, a tetrahedron can be folded from a single sheet of paper. It has two such nets. For any tetrahedron there exists a sphere (called the circumsphere) on which all four vertices lie, and another sphere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angle Strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energies chan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)
