Taxol Total Synthesis Wender Target on:
[Wikipedia]
[Google]
[Amazon]
Paclitaxel (PTX), sold under the brand name Taxol among others, is a chemotherapy medication used to treat a number of types of cancer. This includes ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi's sarcoma,
/ref> It is on the World Health Organization's List of Essential Medicines. It has been made from precursors, and more recently through
." '' Food and Drug Administration.'' January 7, 2005. Retrieved on March 9, 2007. It has since been approved for locally advanced or metastatic non-small cell lung cancer and metastatic
." '' MEDLINE.'' Last Reviewed 09/01/2008. Accessed 10-2-21.
 Paclitaxel is one of several
Paclitaxel is one of several
 Taxol is a tetracyclic diterpene, and the biosynthesis of diterpenes starts with a FPP molecule being elongated by the addition of an IPP molecule in order to form geranylgeranyl diphosphate (
Taxol is a tetracyclic diterpene, and the biosynthesis of diterpenes starts with a FPP molecule being elongated by the addition of an IPP molecule in order to form geranylgeranyl diphosphate (
Image:Taxol.jpg,
''Molecule of the Month: TAXOL''
by Neil Edwards, University of Bristol.
''A Tale of Taxol''
from
cervical cancer
Cervical cancer is a cancer arising from the cervix. It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body. Early on, typically no symptoms are seen. Later symptoms may include abnormal ...
, and pancreatic cancer
Pancreatic cancer arises when cell (biology), cells in the pancreas, a glandular organ behind the stomach, begin to multiply out of control and form a Neoplasm, mass. These cancerous cells have the malignant, ability to invade other parts of t ...
. It is administered by intravenous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrie ...
injection. There is also an albumin-bound formulation.
Common side effects include hair loss, bone marrow suppression, numbness, allergic reactions, muscle pains, and diarrhea. Other serious side effects include heart problems, increased risk of infection, and lung inflammation
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either si ...
. There are concerns that use during pregnancy may cause birth defects. Paclitaxel is in the taxane family of medications. It works by interference with the normal function of microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 an ...
s during cell division.
Paclitaxel was first isolated in 1971 from the Pacific yew
''Taxus brevifolia'', the Pacific yew or western yew, is a species of tree in the yew family Taxaceae native to the Pacific Northwest of North America. It is a small evergreen conifer, thriving in moisture and otherwise tending to take the form o ...
and approved for medical use in 1993.Wayback machine/ref> It is on the World Health Organization's List of Essential Medicines. It has been made from precursors, and more recently through
cell culture
Cell culture or tissue culture is the process by which cells are grown under controlled conditions, generally outside of their natural environment. The term "tissue culture" was coined by American pathologist Montrose Thomas Burrows. This te ...
.
Medical use
Paclitaxel is approved in the UK for ovarian, breast, lung, bladder, prostate,melanoma
Melanoma, also redundantly known as malignant melanoma, is a type of skin cancer that develops from the pigment-producing cells known as melanocytes. Melanomas typically occur in the skin, but may rarely occur in the mouth, intestines, or eye ( ...
, esophageal, and other types of solid tumor cancers as well as Kaposi's sarcoma.
It is recommended in National Institute for Health and Care Excellence (NICE) guidance of June 2001 that it should be used for non-small-cell lung cancer in patients unsuitable for curative treatment, and in first-line and second-line treatment of ovarian cancer. In September 2001, NICE recommended paclitaxel should be available for the treatment of advanced breast cancer after the failure of anthracyclic chemotherapy, but that its first-line use should be limited to clinical trials. In September 2006, NICE recommended paclitaxel should ''not'' be used in the adjuvant treatment In pharmacology, an adjuvant is a drug or other substance, or a combination of substances, that is used to increase the efficacy or potency of certain drugs. Specifically, the term can refer to:
* Adjuvant therapy in cancer management
* Analgesi ...
of early node-positive breast cancer. In 2018, it is approved in the United States for the treatment of breast, pancreatic, ovarian, Kaposi's sarcoma and non-small-cell lung cancers.Product Information: TAXOL(R) IV injection, paclitaxel IV injection. Bristol-Myers Squibb Company, Princeton, NJ, 2013. Accessed from https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020262s051lbl.pdf on 4 October 2018.
Similar compounds
Albumin-bound paclitaxel (trade name Abraxane, also called nab-paclitaxel) is an alternative formulation where paclitaxel is bound to albumin nanoparticles. Much of the clinical toxicity of paclitaxel is associated with the solvent Cremophor EL in which it is dissolved for delivery. Abraxis BioScience developed Abraxane, in which paclitaxel is bonded to albumin as an alternative delivery agent to the often toxic solvent delivery method. This was approved by the FDA in January 2005 for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within six months of adjuvant chemotherapy.Abraxane Drug Information." '' Food and Drug Administration.'' January 7, 2005. Retrieved on March 9, 2007. It has since been approved for locally advanced or metastatic non-small cell lung cancer and metastatic
adenocarcinoma of the pancreas
Pancreatic cancer arises when cells in the pancreas, a glandular organ behind the stomach, begin to multiply out of control and form a mass. These cancerous cells have the ability to invade other parts of the body. A number of types of pancr ...
as well.Product Information: ABRAXANE(R) intravenous injection suspension, paclitaxel protein-bound particles intravenous injection suspension. Celgene Corporation (per FDA), Summit, NJ, 2018. Accessed from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021660s045lbl.pdf on 4 October 2018.
Synthetic approaches to paclitaxel production led to the development of docetaxel. Docetaxel has a similar set of clinical uses to paclitaxel, and it is marketed under the brand name Taxotere.
Taxanes, including paclitaxel, 10-deacetylbaccatin III
10-Deacetylbaccatins are a series of closely related natural organic compounds isolated from the yew tree (Genera ''Taxus''). 10-Deacetylbaccatin III is a precursor to the anti-cancer drug docetaxel (Taxotere).
10-deacetylbaccatin III 10-O-acet ...
, baccatin III, paclitaxel C, and 7-epipaclitaxel, have been found in the leaves and shells of hazel
The hazel (''Corylus'') is a genus of deciduous trees and large shrubs native to the temperate Northern Hemisphere. The genus is usually placed in the birch family Betulaceae,Germplasmgobills Information Network''Corylus''Rushforth, K. (1999). ...
. The finding of these compounds in shells, which are considered discarded material and are mass-produced by many food industries, is of interest for the future availability of paclitaxel.
Restenosis
Paclitaxel is used as an antiproliferative agent for the prevention of restenosis (recurrent narrowing) of coronary and peripheral stents; locally delivered to the wall of the artery, a paclitaxel coating limits the growth of neointima (scar tissue) within stents. Paclitaxel drug-eluting stents for coronary artery placement are sold under the trade name Taxus by Boston Scientific in the United States. Paclitaxel drug-eluting stents for femoropopliteal artery placement are also available.Side effects
Common side effects include nausea and vomiting, loss of appetite, change in taste, thinned or brittle hair, pain in the joints of the arms or legs lasting two to three days, changes in the color of the nails, and tingling in the hands or toes. More serious side effects such as unusual bruising or bleeding, pain, redness or swelling at the injection site, hand-foot syndrome, change in normal bowel habits for more than two days, fever, chills, cough, sore throat, difficulty swallowing, dizziness, shortness of breath, severe exhaustion, skin rash, facial flushing, female infertility by ovarian damage, andchest pain
Chest pain is pain or discomfort in the chest, typically the front of the chest. It may be described as sharp, dull, pressure, heaviness or squeezing. Associated symptoms may include pain in the shoulder, arm, upper abdomen, or jaw, along with n ...
can also occur. Neuropathy
Peripheral neuropathy, often shortened to neuropathy, is a general term describing disease affecting the peripheral nerves, meaning nerves beyond the brain and spinal cord. Damage to peripheral nerves may impair sensation, movement, gland, or o ...
may also occur.
Dexamethasone is given prior to paclitaxel infusion to mitigate some of the side effects.
A number of these side effects are associated with the excipient used, Cremophor EL, a polyoxyethylated castor oil. Allergies to cyclosporine, teniposide
Teniposide (trade name Vumon) is a chemotherapeutic medication used in the treatment of childhood acute lymphocytic leukemia (ALL), Hodgkin's lymphoma, certain brain tumours, and other types of cancer. It is in a class of drugs known as podophyllot ...
, and other drugs delivered in polyoxyethylated castor oil may increase the risk of adverse reactions to paclitaxel.Medline Plus Entry for Paclitaxel Injection." '' MEDLINE.'' Last Reviewed 09/01/2008. Accessed 10-2-21.
Mechanism of action
 Paclitaxel is one of several
Paclitaxel is one of several cytoskeletal drugs
Cytoskeletal drugs are small molecules that interact with actin or tubulin. These drugs can act on the cytoskeletal components within a cell in three main ways. Some cytoskeletal drugs stabilize a component of the cytoskeleton, such as taxol, which ...
that target tubulin. Paclitaxel-treated cells have defects in mitotic spindle assembly, chromosome segregation, and cell division. Unlike other tubulin-targeting drugs, such as colchicine, that inhibit microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 an ...
assembly, paclitaxel stabilizes the microtubule polymer and protects it from disassembly. Chromosomes are thus unable to achieve a metaphase spindle configuration. This blocks the progression of mitosis
In cell biology, mitosis () is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis gives rise to genetically identical cells in which the total number of chromosomes is mainta ...
and prolonged activation of the mitotic checkpoint
Cell cycle checkpoints are control mechanisms in the eukaryotic cell cycle which ensure its proper progression. Each checkpoint serves as a potential termination point along the cell cycle, during which the conditions of the cell are assessed, wi ...
triggers apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
or reversion to the G0-phase of the cell cycle without cell division.
The ability of paclitaxel to inhibit spindle function is generally attributed to its suppression of microtubule dynamics, but other studies have demonstrated that suppression of dynamics occurs at concentrations lower than those needed to block mitosis. At the higher therapeutic concentrations, paclitaxel appears to suppress microtubule detachment from centrosomes, a process normally activated during mitosis. Paclitaxel binds to the beta-tubulin subunits of microtubules.
Chemistry
The nomenclature for paclitaxel is structured on atetracyclic
Tetracyclics are cyclic chemical compounds that contain four interconnected rings of atoms, e.g. Tröger's base.
They have various pharmaceutical
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or s ...
17-atom skeleton. There are a total of 11 stereocenters. The active stereoisomer is (−)-paclitaxel (shown here).
}
Production
Bark processing
From 1967 to 1993, almost all paclitaxel produced was derived from bark of the Pacific yew, '' Taxus brevifolia'', the harvesting of which kills the tree in the process. The processes used were descendants of the original isolation method of Monroe Wall and Mansukh Wani; by 1987, the U.S. National Cancer Institute (NCI) had contracted Hauser Chemical Research ofBoulder, Colorado
Boulder is a home rule city that is the county seat and most populous municipality of Boulder County, Colorado, United States. The city population was 108,250 at the 2020 United States census, making it the 12th most populous city in Color ...
, to handle bark on the scale needed for phase II and III trials. While both the size of the wild population of the Pacific yew and the magnitude of the eventual demand for paclitaxel were uncertain, it was clear that an alternative, sustainable source of the natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
would be needed. Initial attempts to broaden its sourcing used needles from the tree, or material from other related '' Taxus'' species, including cultivated ones, but these attempts were challenged by the relatively low and often highly variable yields obtained. Early in the 1990s, coincident with increased sensitivity to the ecology of the forests of the Pacific Northwest, paclitaxel was successfully extracted on a clinically useful scale from these sources.
Semisynthesis
Concurrently, synthetic chemists in the U.S. and France had been interested in paclitaxel, beginning in the late 1970s. As noted, by 1992 extensive efforts were underway to accomplish the total synthesis of paclitaxel, efforts motivated by the desire to generate new chemical understanding rather than to achieve practical commercial production. In contrast, the French group ofPierre Potier
Pierre Potier (22 August 1934 – 3 February 2006) was a French pharmacist as well as a chemist. He held the position of Director of the Institut de Chimie des Substances Naturelles (1974 to 2000), as well as a teaching position at the Muséum ...
at the Centre national de la recherche scientifique (CNRS) addressed the matter of overall process yield, showing that it was feasible to isolate relatively large quantities of the compound 10-deacetylbaccatin from the European yew, '' Taxus baccata'', which grew on the CNRS campus and whose needles were available in large quantity. By virtue of its structure, 10-deacetylbaccatin was seen as a viable starting material for a short semisynthesis to produce paclitaxel. By 1988, Poitier and collaborators had published a semisynthetic route from needles of the European yew to paclitaxel.
The view of the NCI, however, was that even this route was not practical. The group of Robert A. Holton
Robert A. Holton (born 1944) is an American academic chemist who is known for his work regarding the chemical synthesis for Taxol (known as the Holton Taxol total synthesis), a widely utilized and highly effective anti-cancer drug. He is a Profes ...
had also pursued a practical semisynthetic production route; by late 1989, Holton's group had developed a semisynthetic route to paclitaxel with twice the yield of the Potier process. Florida State University
Florida State University (FSU) is a public research university in Tallahassee, Florida. It is a senior member of the State University System of Florida. Founded in 1851, it is located on the oldest continuous site of higher education in the st ...
, where Holton worked, signed a deal with Bristol-Myers Squibb (BMS) to license their semisynthesis and future patents. In 1992, Holton patented an improved process with an 80% yield, and BMS took the process in-house and started to manufacture paclitaxel in Ireland from 10-deacetylbaccatin isolated from the needles of the European yew. In early 1993, BMS announced that it would cease reliance on Pacific yew bark by the end of 1995, effectively terminating ecological controversy over its use. This announcement also made good their commitment to develop an alternative supply route, made to the NCI in their cooperative research and development agreement (CRADA) application of 1989.
As of 2013, BMS was using the semisynthetic method utilizing needles from the European yew to produce paclitaxel. Another company which worked with BMS until 2012, Phyton Biotech, Inc., uses plant cell fermentation (PCF) technology. By cultivating a specific ''Taxus'' cell line
An immortalised cell line is a population of cells from a multicellular organism which would normally not proliferate indefinitely but, due to mutation, have evaded normal cellular senescence and instead can keep undergoing division. The cell ...
in fermentation tanks, they no longer need ongoing sourcing of material from actual yew tree plantations. Paclitaxel is then captured directly from the suspension broth by a resin allowing concentration to highly enriched powder containing about 40% paclitaxel. The compound is then purified by one chromatographic step followed by crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposi ...
. Compared to the semisynthesis method, PCF eliminates the need for many hazardous chemicals and saves a considerable amount of energy.
In 1993, paclitaxel was discovered as a natural product in a newly described endophytic fungus living in the yew tree. It has since been reported in a number of other endophytic fungi, including ''Nodulisporium sylviforme'', ''Alternaria taxi'', ''Cladosporium cladosporioides'' MD2, '' Metarhizium anisopliae'', ''Aspergillus candidus'' MD3, ''Mucor rouxianus'', ''Chaetomella raphigera'', ''Phyllosticta tabernaemontanae'', '' Phomopsis'', ''Pestalotiopsis pauciseta'', ''Phyllosticta citricarpa
Citrus black spot is a fungal disease caused by Phyllosticta citricarpa'(previously known as ''Guignardia citricarpa)''. This Ascomycete fungus affects citrus plants throughout subtropical climates, causing a reduction in both fruit quantity and q ...
'', '' Podocarpus'' sp., '' Fusarium solani'', ''Pestalotiopsis terminaliae'', ''Pestalotiopsis breviseta'', ''Botryodiplodia theobromae'', ''Gliocladium'' sp., ''Alternaria alternata'' var. ''monosporus'', '' Cladosporium cladosporioides'', ''Nigrospora'' sp., ''Pestalotiopsis versicolor
''Pestalotiopsis versicolor'' is a plant pathogen infecting avocado
The avocado (''Persea americana'') is a medium-sized, evergreen tree in the laurel family (Lauraceae). It is native to the Americas and was first domesticated by Mesoamer ...
'', and ''Taxomyces andreanae''. However, there has been contradictory evidence for its production by endophytes, with other studies finding independent production is unlikely.
Biosynthesis
 Taxol is a tetracyclic diterpene, and the biosynthesis of diterpenes starts with a FPP molecule being elongated by the addition of an IPP molecule in order to form geranylgeranyl diphosphate (
Taxol is a tetracyclic diterpene, and the biosynthesis of diterpenes starts with a FPP molecule being elongated by the addition of an IPP molecule in order to form geranylgeranyl diphosphate (GGPP
Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and di terpenoids. It is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls.
It is also a precursor to geranylgeranylated proteins, ...
). The biosynthesis of Taxol contains nineteen steps. These 19 steps can be considered in several steps, with the first step being the formation of the taxane skeleton, which then undergoes a series of oxygenations. Following the oxygenations, two acetylations and a benzoylation occur on the intermediate. The oxygenation of the taxane core is believed to occur on C5 and C10, C2 and C9, C13 followed by C7, and a C1 hydroxylation later on in the pathway. Later in the pathway, an oxidation at C9 forms a ketone functional group and an oxetane, forming the intermediate baccatin III. The final steps of the pathway include the formation of a C13-side chain which is attached to baccatin III. The biosynthesis of Taxol is illustrated in more detail in the figure, with steps 1-7 all occurring in the enzyme taxadiene synthase (TS on the figure). Taxol's biosynthesis begins with E,E,E-GGPP losing pyrophosphate via an SN1 mechanism (step 1 in the figure). The double-bond attacks the cation via electrophilic addition, yielding a tertiary cation and creating the first ring closure (step 2). Another electrophilic attack occurs, further cyclizing the structure by creating the first 6-membered ring and creating another tertiary cation (step 3). An intramolecular proton transfer occurs, attacking the verticillyl cation (step 4) and creating a double bond, yielding a tertiary cation. An electrophilic cyclization occurs in step 5, and an intramolecular proton transfer attacks the taxenyl cation (step 6). This forms the fused ring structure intermediate known as taxadiene. Taxadiene then undergoes a series of 10 oxidations via NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NAD ...
, forming the intermediate taxadiene-5α-acetoxy-10β-ol (multiple steps later in the figure). A series of hydroxylations and esterficiations occur, forming the intermediate 10-deacetyl-baccatin III, which undergoes a further series of esterifications and a side-chain hydroxylation. This finally yields the product taxol.
Total synthesis
By 1992, at least thirty academic research teams globally were working to achieve a total synthesis of thisnatural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
, with the synthesis proceeding from simple natural products and other readily available starting materials. This total synthesis effort was motivated primarily by the desire to generate new chemical understanding, rather than with an expectation of the practical commercial production of paclitaxel. The first laboratories to complete the total synthesis from much less complex starting materials were the research groups of Robert A. Holton
Robert A. Holton (born 1944) is an American academic chemist who is known for his work regarding the chemical synthesis for Taxol (known as the Holton Taxol total synthesis), a widely utilized and highly effective anti-cancer drug. He is a Profes ...
, who had the first article to be accepted for publication, and of K. C. Nicolaou
Kyriacos Costa Nicolaou ( el, Κυριάκος Κ. Νικολάου; born July 5, 1946) is a Cypriot-American chemist known for his research in the area of natural products total synthesis. He is currently Harry C. and Olga K. Wiess Professor of ...
who had the first article to appear in print (by a week, on 7 February 1994). Though the Holton submission preceded the Nicolaou by a month (21 December 1993 versus 24 January 1994), the near coincidence of the publications arising from each of these massive, multiyear efforts—11–18 authors appearing on each of the February 1994 publications—has led the ending of the race to be termed a "tie" or a "photo finish", though each group has argued that their synthetic strategy and tactics were superior.
As of 2006, five additional research groups had reported successful total syntheses of paclitaxel: Wender et al. in 1997, and Kuwajima et al. and Mukaiyama et al. in 1998 with further linear syntheses, and Danishefsky et al. in 1996 and Takahashi et al. in 2006 with further convergent syntheses. As of that date, all strategies had aimed to prepare a 10-deacetylbaccatin-type core containing the ABCD ring system, followed generally by last stage addition of the "tail" to the 13-hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy g ...
.
While the "political climate surrounding aclitaxeland he Pacific yew
He or HE may refer to:
Language
* He (pronoun), an English pronoun
* He (kana), the romanization of the Japanese kana へ
* He (letter), the fifth letter of many Semitic alphabets
* He (Cyrillic), a letter of the Cyrillic script called ''He'' ...
in the early 1990s ... helped bolster link between total synthesis and the aclitaxelsupply problem," and though total synthesis activities were a requisite to explore the structure-activity relationships of paclitaxel via generation of analogs for testing, the total synthesis efforts were never seen "as a serious commercial route" to provide significant quantities of the natural product for medical testing or therapeutic use.
History
The discovery of paclitaxel began in 1962 as a result of a NCI-funded screening program. A number of years later it was isolated from the bark of the Pacific yew, ''Taxus brevifolia'', hence its name "taxol". The discovery was made by Monroe E. Wall andMansukh C. Wani
Mansukh C. Wani, (died 2020), was a principal scientist (emeritus) at the Research Triangle Institute in North Carolina. He was co-discoverer of Taxol and camptothecin, two anti-cancer drugs considered standard in the treatment to fight ovarian, ...
at the Research Triangle Institute, Research Triangle Park
Research Triangle Park (RTP) is the largest research park in the United States, occupying in North Carolina and hosting more than 300 companies and 65,000 workers.
The facility is named for its location relative to the three surrounding cities ...
, North Carolina, in 1971. These scientists isolated the natural product from the bark of the Pacific yew tree, determined its structure and named it "taxol", and arranged for its first biological testing. The compound was then developed commercially by BMS, who had the generic name assigned as "paclitaxel".
Plant screening program
In 1955, the NCI in the United States set up the Cancer Chemotherapy National Service Center (CCNSC) to act as a public screening center for anticancer activity in compounds submitted by external institutions and companies. Although the majority of compounds screened were of synthetic origin, one chemist, Jonathan Hartwell, who was employed there from 1958 onwards, had experience with natural product derived compounds, and began a plant screening operation. After some years of informal arrangements, in July 1960, the NCI commissioned the United States Department of Agriculture (USDA) botanists to collect samples from about 1,000 plant species per year. On 21 August 1962, one of those botanists, Arthur S. Barclay, collected bark from a single Pacific yew tree in a forest north of the town ofPackwood, Washington
Packwood is an unincorporated community and census-designated place (CDP) located in easternmost Lewis County, Washington, in the United States. As of the 2020 census, the CDP had a population of 319, while the town and surrounding Packwood com ...
, as part of a four-month trip to collect material from over 200 different species. The material was then processed by a number of specialist CCNSC subcontractors, and one of the tree's samples was found to be cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa'').
Cell physiology
Treating cells ...
in a cellular assay on 22 May 1964.
Accordingly, in late 1964 or early 1965, the fractionation and isolation laboratory run by Monroe E. Wall in Research Triangle Park, North Carolina, began work on fresh ''Taxus'' samples, isolating the active ingredient in September 1966 and announcing their findings at an April 1967 American Chemical Society meeting in Miami Beach
Miami Beach is a coastal resort city in Miami-Dade County, Florida. It was incorporated on March 26, 1915. The municipality is located on natural and man-made barrier islands between the Atlantic Ocean and Biscayne Bay, the latter of which sep ...
. They named the pure compound taxol in June 1967. Wall and his colleague Wani published their results, including the chemical structure, in 1971.
The NCI continued to commission work to collect more ''Taxus'' bark and to isolate increasing quantities of taxol. By 1969, of crude extract had been isolated from almost of bark, although this ultimately yielded only of pure material, but for several years, no use was made of the compound by the NCI. In 1975, it was shown to be active in another '' in vitro'' system; two years later, a new department head reviewed the data and finally recommended taxol be moved on to the next stage in the discovery process. This required increasing quantities of purified taxol, up to , and in 1977 a further request for of bark was made.
In 1978, two NCI researchers published a report showing taxol was mildly effective in leukaemic mice. In November 1978, taxol was shown to be effective in xenograft studies. Meanwhile, taxol began to be well known in the cell biology, as well as the cancer community, with a publication in early 1979 by Susan B. Horwitz
Susan Beth Horwitz (January 6, 1955 – June 11, 2014) was an American computer scientist noted for her research on
programming languages and software engineering, and in particular on program slicing and
dataflow-analysis. She had several ...
, a molecular pharmacologist at Albert Einstein College of Medicine, showing taxol had a previously unknown mechanism of action involving the stabilization of microtubules. Together with formulation problems, this increased interest from researchers meant that, by 1980, the NCI envisaged needing to collect of bark. Animal toxicology studies were complete by June 1982, and in November NCI applied for the IND necessary to begin clinical trials in humans.
Early clinical trials, supply and the transfer to BMS
Phase I Phase 1, Phase I or Phase One may refer to:
Media
* Marvel Cinematic Universe: Phase One, six American superhero films from 2008–2012
* ''Phase One'' (Art Ensemble of Chicago album), 1971
* ''Phase One'' (Saga album), 1998
* ''Phase One'', r ...
clinical trials began in April 1984, and the decision to start Phase II Phase II, Phase 2 or Phase Two may refer to:
Media
* Marvel Cinematic Universe: Phase Two, six American superhero films from 2013–2015
* ''Star Trek: Phase II'', an unrealized television series based on the characters of Gene Roddenberry's ''S ...
trials was made a year later. These larger trials needed more bark and collection of a further 12,000 pounds was commissioned, which enabled some phase II trials to begin by the end of 1986. But by then it was recognized that the demand for taxol might be substantial and that more than 60,000 pounds of bark might be needed as a minimum. This unprecedentedly large amount brought ecological concerns about the impact on yew populations into focus for the first time, as local politicians and foresters expressed unease at the program.
The first public report from a phase II trial in May 1988 showed promising effects in melanoma and refractory ovarian cancer. At this point, Gordon Cragg of the NCI's Natural Product Branch calculated the synthesis of enough taxol to treat all the ovarian cancer and melanoma cases in the US would require the destruction of 360,000 trees annually. For the first time, serious consideration was given to the problem of supply.
Because of the practical and, in particular, the financial scale of the program needed, the NCI decided to seek association with a pharmaceutical company, and in August 1989, it published a Cooperative Research and Development Agreement (CRADA) offering its current stock and supply from current bark stocks, and proprietary access to the data so far collected, to a company willing to commit to providing the funds to collect further raw material, isolate taxol, and fund a large proportion of clinical trials. In the words of Goodman and Welsh, authors of a substantial scholarly book on taxol, "The NCI was thinking, not of collaboration, ... but of a hand-over of taxol (and its problems)".
Although the offer was widely advertised, only four companies responded to the CRADA, including the American firm Bristol-Myers Squibb (BMS),
which was selected as the partner in December 1989. The choice of BMS later became controversial and was the subject of Congressional hearings in 1991 and 1992. While it seems clear the NCI had little choice but to seek a commercial partner, there was also controversy about the terms of the deal, eventually leading to a report by the General Accounting Office in 2003, which concluded the NIH had failed to ensure value for money. In related CRADAs with the USDA and Department of the Interior
The United States Department of the Interior (DOI) is one of the executive departments of the U.S. federal government headquartered at the Main Interior Building, located at 1849 C Street NW in Washington, D.C. It is responsible for the mana ...
, Bristol-Myers Squibb was given exclusive first refusal on all Federal supplies of ''Taxus brevifolia''.
This exclusive contract lead to some criticism for giving BMS a "cancer monopoly".
Eighteen months after the CRADA, BMS filed a new drug application (NDA), which was given FDA approval at the very end of 1992.
Although there was no patent on the compound, the provisions of the Waxman-Hatch Act gave Bristol-Myers Squibb five years exclusive marketing rights.
In 1990, BMS applied to trademark the name taxol as ''Taxol(R)''. This was controversially approved in 1992. At the same time, paclitaxel replaced taxol as the generic ( INN) name of the compound. Critics, including the journal '' Nature'', argued the name taxol had been used for more than two decades and in more than 600 scientific articles and suggested the trademark should not have been awarded and the BMS should renounce its rights to it. BMS argued changing the name would cause confusion among oncologists and possibly endanger the health of patients. BMS has continued to defend its rights to the name in the courts.
BMS has also been criticized for misrepresentation by Goodman and Walsh, who quote from a company report saying "It was not until 1971 that ... testing ... enabled the isolation of paclitaxel, initially described as 'compound 17". This quote is, strictly speaking, accurate: the objection seems to be that this misleadingly neglects to explain that it was the scientist doing the isolation who named the compound taxol and it was not referred to in any other way for more than twenty years. Annual sales peaked in 2000, reaching US$1.6 billion; paclitaxel is now available in generic form.
Society and culture
, the cost to the NHS per patient in early breast cancer, assuming four cycles of treatment, was about £4,000 (approx. $6,000).Research
Caffeine has been speculated to inhibit paclitaxel-induced apoptosis in colorectal cancer cells. Aside from its direct clinical use, paclitaxel is used extensively in biological and biomedical research as amicrotubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 an ...
stabilizer. In general, in vitro assays involving microtubules, such as motility assays, rely on paclitaxel to maintain microtubule integrity in the absence of the various nucleating factors and other stabilizing elements found in the cell. For example, it is used for in vitro tests of drugs that aim to alter the behavior of microtubule motor proteins, or for studies of mutant motor proteins. Moreover, Paclitaxel has been used in vitro to inhibit insulin fibrillation; in a molar ratio of 10:1 (insulin:paclitaxel), it hindered insulin fibrillation near 70%. Iso-thermal titration calorimetry (ITC) findings indicated a spontaneous tendency of paclitaxel to interact with insulin through hydrogen bonds and van der Waal's forces. Also, the inhibitory role of paclitaxel is attributed to its impact on the colloidal stability of protein solution, as it was observed that paclitaxel inhibited lysozyme fibrillation by inducing the formation of "off-pathway" oligomeric intermediates and increasing the colloidal stability subsequently. Paclitaxel is sometimes used for in vivo studies as well; it can be fed to test organisms, such as fruit flies, or injected into individual cells, to inhibit microtubule disassembly or to increase the number of microtubules in the cell. Paclitaxel induces remyelination in a demyelinating mouse in vivo and inhibits hPAD2 in vitro though its methyl ester side chain. Angiotech Pharmaceuticals Inc. began phase II clinical trials in 1999 as a multiple sclerosis treatment but in 2002, reported that the results showed no statistical significance.
In 2016 ''in vitro'' multi-drug resistant mouse tumor cells were treated with paclitaxel encased in exosomes. Doses 98% less than common dosing had the same effect. Also, dye-marked exosomes were able to mark tumor cells, potentially aiding in diagnosis.
Additional images
Space-filling model
In chemistry, a space-filling model, also known as a ''calotte model'', is a type of three-dimensional (3D) molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to- ...
of paclitaxel
Image:Taxol.gif, Rotating paclitaxel molecule model
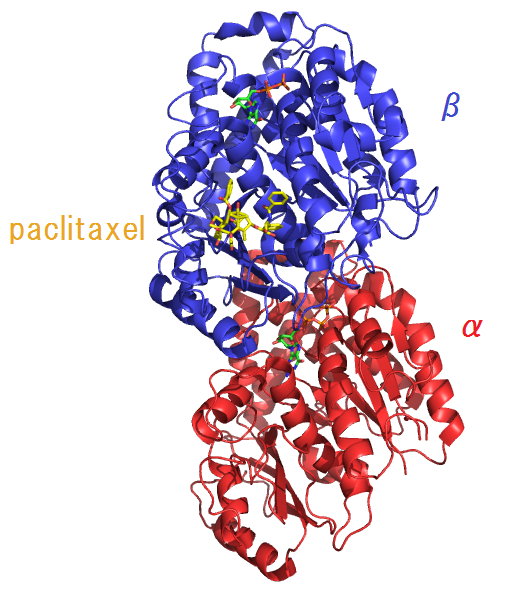
Image:Paclitaxel JMolBiol 2001 1045.jpg, Crystal structure of paclitaxel
Image:Taxol total charge surface.gif, Total charge surface of taxol. Minimum energy conformation.
References
Further reading
*External links
* * *''Molecule of the Month: TAXOL''
by Neil Edwards, University of Bristol.
''A Tale of Taxol''
from
Florida State University
Florida State University (FSU) is a public research university in Tallahassee, Florida. It is a senior member of the State University System of Florida. Founded in 1851, it is located on the oldest continuous site of higher education in the st ...
.
*
{{Authority control
Bristol Myers Squibb
Microtubule inhibitors
Mitotic inhibitors
Benzoate esters
Carboxylate esters
Benzamides
Secondary alcohols
Tertiary alcohols
Ketones
Acetate esters
Taxanes
Pregnane X receptor agonists
World Health Organization essential medicines
Wikipedia medicine articles ready to translate
Plant toxins