Surface Potential on:
[Wikipedia]
[Google]
[Amazon]
Surface charge is a two-dimensional surface with non-zero
The Helmholtz model, while a good foundation for the description of the interface does not take into account several important factors: diffusion/mixing in solution, the possibility of adsorption on to the surface and the interaction between solvent dipole moments and the electrode.
 Gouy-Chapman theory describes the effect of a static surface charge on a surface's potential. " Gouy suggested that interfacial potential at the charged surface could be attributed to the presence of a number of ions of given charge attached to its surface, and to an equal number of ions of opposite charge in the solution." A positive surface charge will form a double layer, since negative ions in solution tend to balance the positive surface charge. Counter ions are not rigidly held, but tend to diffuse into the liquid phase until the counter potential set up by their departure restricts this tendency. The kinetic energy of the counter ions will, in part, affect the thickness of the resulting diffuse double layer. The relation between C, the counter ion concentration at the surface, and , the counter ion concentration in the external solution, is the Boltzmann factor:
where ''z'' is the charge on the ion, ''e'' is the charge of a proton, ''k''B is the
Gouy-Chapman theory describes the effect of a static surface charge on a surface's potential. " Gouy suggested that interfacial potential at the charged surface could be attributed to the presence of a number of ions of given charge attached to its surface, and to an equal number of ions of opposite charge in the solution." A positive surface charge will form a double layer, since negative ions in solution tend to balance the positive surface charge. Counter ions are not rigidly held, but tend to diffuse into the liquid phase until the counter potential set up by their departure restricts this tendency. The kinetic energy of the counter ions will, in part, affect the thickness of the resulting diffuse double layer. The relation between C, the counter ion concentration at the surface, and , the counter ion concentration in the external solution, is the Boltzmann factor:
where ''z'' is the charge on the ion, ''e'' is the charge of a proton, ''k''B is the

electric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respe ...
. These electric charges are constrained on this 2-D surface, and surface charge density, measured in coulombs per square meter (C•m−2), is used to describe the charge distribution on the surface. The electric potential
The electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as the amount of work energy needed to move a unit of electric charge from a reference point to the specific point in ...
is continuous
Continuity or continuous may refer to:
Mathematics
* Continuity (mathematics), the opposing concept to discreteness; common examples include
** Continuous probability distribution or random variable in probability and statistics
** Continuous ...
across a surface charge and the electric field is discontinuous, but not infinite; this is unless the surface charge consists of a dipole layer. In comparison, the potential and electric field both diverge at any point charge
A point particle (ideal particle or point-like particle, often spelled pointlike particle) is an idealization of particles heavily used in physics. Its defining feature is that it lacks spatial extension; being dimensionless, it does not take u ...
or linear charge.
In physics, at equilibrium, an ideal conductor has no charge on its interior; instead, the entirety of the charge of the conductor resides on the surface. However, this only applies to the ideal case of infinite electrical conductivity; The majority of the charge of an actual conductor resides within the skin depth
Skin effect is the tendency of an alternating electric current (AC) to become distributed within a conductor such that the current density is largest near the surface of the conductor and decreases exponentially with greater depths in the co ...
of the conductor's surface. For dielectric
In electromagnetism, a dielectric (or dielectric medium) is an electrical insulator that can be polarised by an applied electric field. When a dielectric material is placed in an electric field, electric charges do not flow through the mate ...
materials, upon the application of an external electric field, the positive charges and negative charges in the material will slightly move in opposite directions, resulting in polarization density
In classical electromagnetism, polarization density (or electric polarization, or simply polarization) is the vector field that expresses the density of permanent or induced electric dipole moments in a dielectric material. When a dielectric is ...
in the bulk body and bound charge
In classical electromagnetism, polarization density (or electric polarization, or simply polarization) is the vector field that expresses the density of permanent or induced electric dipole moments in a dielectric material. When a dielectric is ...
at the surface.
In chemistry, there are many different processes which can lead to a surface being charged, including adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
of ions, protonation/deprotonation, and, as discussed above, the application of an external electric field. Surface charge emits an electric field, which causes particle repulsion and attraction, affecting many colloidal properties.
Surface charge practically always appears on the particle surface when it is placed into a fluid. Most fluids contain ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
, positive (cations
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by con ...
) and negative (anions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
). These ions interact with the object surface. This interaction might lead to the adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
of some of them onto the surface. If the number of adsorbed cations exceeds the number of adsorbed anions, the surface would have a net positive electric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respe ...
.
Dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts ...
of the surface chemical group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
is another possible mechanism leading to surface charge.
Density
Surface charge density is defined as the amount ofelectric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respe ...
, q, that is present on a surface of given area, ''A'':
Conductors
According to Gauss’s law, a conductor at equilibrium carrying an applied current has no charge on its interior. Instead, the entirety of the charge of the conductor resides on the surface, and can be expressed by the equation: where E is the electric field caused by the charge on the conductor and is the permittivity of the free space. This equation is only strictly accurate for conductors with infinitely large area, but it provides a good approximation if E is measured at an infinitesimally small Euclidean distance from the surface of the conductor.Colloids and immersed objects
When a surface is immersed in a solution containingelectrolytes
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon di ...
, it develops a net surface charge. This is often because of ionic adsorption. Aqueous solutions universally contain positive and negative ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
(cations
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by con ...
and anions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
, respectively), which interact with partial charge A partial charge is a non-integer charge value when measured in elementary charge units. Partial charge is more commonly called net atomic charge. It is represented by the Greek lowercase letter 𝛿, namely 𝛿− or 𝛿+.
Partial charges are c ...
s on the surface, adsorbing to and thus ionizing the surface and creating a net surface charge. This net charge results in a surface potential which causes the surface to be surrounded by a cloud of counter-ions, which extends from the surface into the solution, and also generally results in repulsion between particles. The larger the partial charges in the material, the more ions are adsorbed to the surface, and the larger the cloud of counter-ions. A solution with a higher concentration of electrolytes also increases the size of the counter-ion cloud. This ion/counterion layer is known as the electric double layer.
A solution's pH can also greatly affect surface charge because functional groups present on the surface of particles can often contain oxygen or nitrogen, two atoms which can be protonated or deprotonated to become charged. Thus, as the concentration of hydrogen ions changes, so does the surface charge of the particles. At a certain pH, the average surface charge will be equal to zero; this is known as the point of zero charge (PZC). A list of common substances and their associated PZCs is shown to the right.
Interfacial potential
An interface is defined as the common boundary formed between two different phases, such as between a solid and gas.Electric potential
The electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as the amount of work energy needed to move a unit of electric charge from a reference point to the specific point in ...
, or charge, is the result of an object's capacity to be moved in an electric field. An interfacial potential is thus defined as a charge located at the common boundary between two phases (for example, an amino acid such as glutamate on the surface of a protein can have its side chain carboxylic acid deprotonated in environments with pH greater than 4.1 to produce a charged amino acid at the surface, which would create an interfacial potential). Interfacial potential is responsible for the formation of the electric double layer, which has a broad range of applications in what is termed electrokinetic phenomena Electrokinetic phenomena are a family of several different effects that occur in heterogeneous fluids, or in porous bodies filled with fluid, or in a fast flow over a flat surface. The term heterogeneous here means a fluid containing particles. Part ...
. The development of the theory of the electric double layer is described below.
Helmholtz
The model dubbed the 'electric double layer' was first introduced byHermann von Helmholtz
Hermann Ludwig Ferdinand von Helmholtz (31 August 1821 – 8 September 1894) was a German physicist and physician who made significant contributions in several scientific fields, particularly hydrodynamic stability. The Helmholtz Associatio ...
. It assumes that a solution is only composed of electrolytes, no reactions occur near the electrode which could transfer electrons, and that the only Van der Waals interactions are present between the ions in solution and the electrode. These interactions arise only due to the charge density associated with the electrode which arises from either an excess or deficiency of electrons at the electrode's surface. To maintain electrical neutrality the charge of the electrode will be balanced by a redistribution of ions close to its surface. The attracted ions thus form a layer balancing the electrode's charge. The closest distance an ion can come to the electrode will be limited to the radius of the ion plus a single solvation sphere around an individual ion. Overall, two layers of charge and a potential drop from the electrode to the edge of the outer layer (outer Helmholtz Plane) are observed.
Given the above description, the Helmholtz model is equivalent in nature to an electrical capacitor with two separated plates of charge, for which a linear potential drop is observed at increasing distance from the plates. The Helmholtz model, while a good foundation for the description of the interface does not take into account several important factors: diffusion/mixing in solution, the possibility of adsorption on to the surface and the interaction between solvent dipole moments and the electrode.
Gouy-Chapman
 Gouy-Chapman theory describes the effect of a static surface charge on a surface's potential. " Gouy suggested that interfacial potential at the charged surface could be attributed to the presence of a number of ions of given charge attached to its surface, and to an equal number of ions of opposite charge in the solution." A positive surface charge will form a double layer, since negative ions in solution tend to balance the positive surface charge. Counter ions are not rigidly held, but tend to diffuse into the liquid phase until the counter potential set up by their departure restricts this tendency. The kinetic energy of the counter ions will, in part, affect the thickness of the resulting diffuse double layer. The relation between C, the counter ion concentration at the surface, and , the counter ion concentration in the external solution, is the Boltzmann factor:
where ''z'' is the charge on the ion, ''e'' is the charge of a proton, ''k''B is the
Gouy-Chapman theory describes the effect of a static surface charge on a surface's potential. " Gouy suggested that interfacial potential at the charged surface could be attributed to the presence of a number of ions of given charge attached to its surface, and to an equal number of ions of opposite charge in the solution." A positive surface charge will form a double layer, since negative ions in solution tend to balance the positive surface charge. Counter ions are not rigidly held, but tend to diffuse into the liquid phase until the counter potential set up by their departure restricts this tendency. The kinetic energy of the counter ions will, in part, affect the thickness of the resulting diffuse double layer. The relation between C, the counter ion concentration at the surface, and , the counter ion concentration in the external solution, is the Boltzmann factor:
where ''z'' is the charge on the ion, ''e'' is the charge of a proton, ''k''B is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, ...
and ''ψ'' is the potential of the charged surface.
This however is inaccurate close to the surface, because it assumes that molar concentration is equal to activity. It also assumes that ions were modeled as point charges and was later modified. An improvement of this theory, known as the modified Gouy-Chapman theory, included the finite size of the ions with respect to their interaction with the surface in the form of a plane of closest approach.
Surface potential
The relation between surface charge and surface potential can be expressed by the Grahame equation, derived from the Gouy-Chapman theory by assuming the electroneutrality condition, which states that the total charge of the double layer must be equal to the negative of the surface charge. Using the one-dimensionalPoisson equation
Poisson's equation is an elliptic partial differential equation of broad utility in theoretical physics. For example, the solution to Poisson's equation is the potential field caused by a given electric charge or mass density distribution; with t ...
and assuming that, at an infinitely great distance, the potential gradient
In physics, chemistry and biology, a potential gradient is the local rate of change of the potential with respect to displacement, i.e. spatial derivative, or gradient. This quantity frequently occurs in equations of physical processes because i ...
is equal to 0, the Grahame equation is obtained:
For the case of lower potentials, can be expanded to , and is defined as the Debye length
In plasmas and electrolytes, the Debye length \lambda_ (also called Debye radius), is a measure of a charge carrier's net electrostatic effect in a solution and how far its electrostatic effect persists. With each Debye length the charges are in ...
. Which leads to the simple expression:

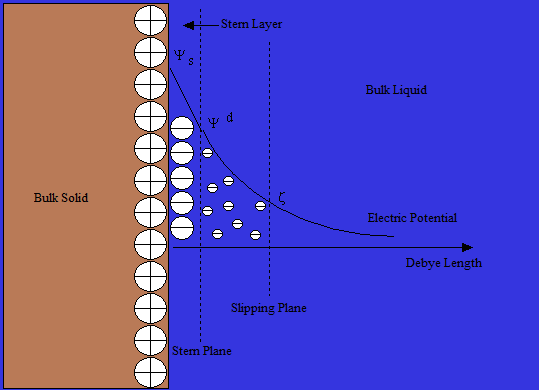
Stern
TheOtto Stern
:''Otto Stern was also the pen name of German women's rights activist Louise Otto-Peters (1819–1895)''.
Otto Stern (; 17 February 1888 – 17 August 1969) was a German-American physicist and Nobel laureate in physics. He was the second most n ...
model of the double layer is essentially a combination of Helmholtz and Gouy-Chapman theories. His theory states that ions do have finite size, so cannot approach the surface closer than a few nanometers. Through a distance known as the Stern Layer, ions can be adsorbed onto the surface up to a point referred to as the slipping plane, where the ions adsorbed meet the bulk liquid. At the slipping plane the potential Ψ has decreased to what is known as the zeta potential
Zeta potential is the electrical potential at the slipping plane. This plane is the interface which separates mobile fluid from fluid that remains attached to the surface.
Zeta potential is a scientific term for electrokinetic potential in coll ...
. Although zeta potential is an intermediate value, it is sometimes considered to be more significant than surface potential as far as electrostatic repulsion is concerned.
Applications
Charged surfaces are extremely important and are used in many applications. For example, solutions of large colloidal particles depend almost entirely on repulsion due to surface charge in order to stay dispersed. If these repulsive forces were to be disrupted, perhaps by the addition of a salt or a polymer, the colloidal particles would no longer be able to sustain suspension and would subsequentlyflocculate
Flocculation, in the field of chemistry, is a process by which colloidal particles come out of suspension to sediment under the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from p ...
.
Electrokinetic phenomena

Electrokinetic phenomena Electrokinetic phenomena are a family of several different effects that occur in heterogeneous fluids, or in porous bodies filled with fluid, or in a fast flow over a flat surface. The term heterogeneous here means a fluid containing particles. Part ...
refers to a variety of effects resulting from an electrical double layer. A noteworthy example is electrophoresis, where a charged particle suspended in a media will move as a result of an applied electrical field. Electrophoresis is widely used in biochemistry to distinguish molecules, such as proteins, based on size and charge. Other examples include electro-osmosis Electroosmotic flow (or electro-osmotic flow, often abbreviated EOF; synonymous with electroosmosis or electroendosmosis) is the motion of liquid induced by an applied potential across a porous material, capillary tube, membrane, microchannel, or an ...
, sedimentation potential, and streaming potential
Streaming media is multimedia that is delivered and consumed in a continuous manner from a source, with little or no intermediate storage in network elements. ''Streaming'' refers to the delivery method of content, rather than the content it ...
.
Proteins
Proteins often have groups present on their surfaces that can be ionized or deionized depending on pH, making it relatively easy to change the surface charge of a protein. This has particularly important ramifications on the activity of proteins that function as enzymes or membrane channels, mainly, that the protein's active site must have the right surface charge in order to be able to bind a specific substrate.Adhesives/coatings
Charged surfaces are often useful in creating surfaces that will not adsorb certain molecules (for example, in order to prevent the adsorption of basic proteins, a positively charged surface should be used). Polymers are very useful in this respect in that they can be functionalized so that they contain ionizable groups, which serve to provide a surface charge when submerged in an aqueous solution.{{Cite journal , last1 = Haselberg , first1 = Rob , last2 = van der Sneppen , first2 = Lineke , last3 = Ariese , first3 = Freek , last4 = Ubachs , first4 = Wim , last5 = Gooijer , first5 = Cees , last6 = de Jong , first6 = Gerhardus J. , last7 = Somsen , first7 = Govert W. , title = Effectiveness of charged non-covalent polymer coatings against protein adsorption to silica surfaces studied by evanescent-wave cavity ring-down spectroscopy and capillary electrophoresis , journal = Analytical Chemistry , volume = 81 , issue = 24 , pages = 10172–10178 , date = 18 Nov 2009 , doi = 10.1021/ac902128n , pmid = 19921852References