|
Electrokinetic Phenomena
Electrokinetic phenomena are a family of several different effects that occur in heterogeneous fluids, or in porous bodies filled with fluid, or in a fast flow over a flat surface. The term heterogeneous here means a fluid containing particles. Particles can be solid, liquid or gas bubbles with sizes on the scale of a micrometer or nanometer.Dukhin, A. S. and Goetz, P. J. ''Characterization of liquids, nano- and micro- particulates and porous bodies using Ultrasound'', Elsevier, 2017 There is a common source of all these effects—the so-called interfacial 'double layer' of charges. Influence of an external force on the diffuse layer generates tangential motion of a fluid with respect to an adjacent charged surface. This force might be electric, pressure gradient, concentration gradient, or gravity. In addition, the moving phase might be either continuous fluid or dispersed phase. Family Various combinations of the driving force and moving phase determine various electrokinetic ef ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterogeneous
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, size, weight, height, distribution, texture, language, income, disease, temperature, radioactivity, architectural design, etc.); one that is heterogeneous is distinctly nonuniform in at least one of these qualities. Heterogeneous Mixtures, in chemistry, is where certain elements are unwillingly combined and, when given the option, will separate. Etymology and spelling The words ''homogeneous'' and ''heterogeneous'' come from Medieval Latin ''homogeneus'' and ''heterogeneus'', from Ancient Greek ὁμογενής (''homogenēs'') and ἑτερογενής (''heterogenēs''), from ὁμός (''homos'', “same”) and ἕτερος (''heteros'', “other, another, different”) respectively, followed by γένος (''genos'', “kind”); - ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
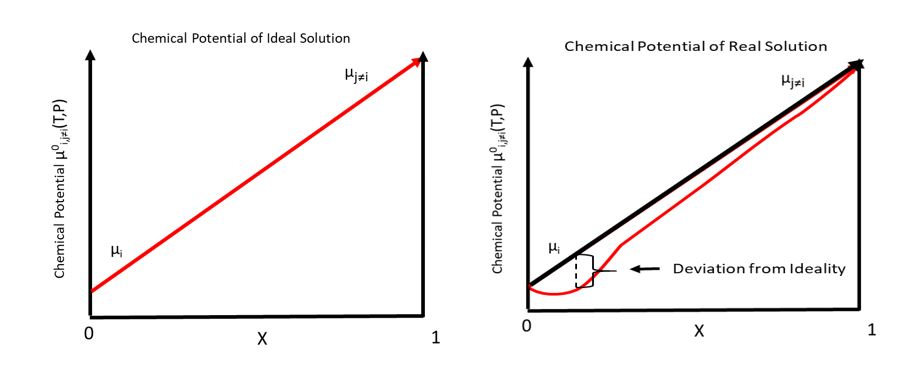
Chemical Potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free energy is at a minimum. In a system in diffusion equilibrium, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cationization Of Cotton
Cationization of cotton is an electro kinetic phenomena ( zeta potential and electro kinetic) for surface charge of cotton. The cotton surface is charged with positive ions. Cationization alters the characterization of the surface of the cotton which allows salt free dyeing and improves the dye ability of cotton. The process involves the chemical reaction of cationic reactive agents with cellulose. Etymology A cation (+) (/ˈkætˌaɪ.ən/), from the Greek word κάτω (káto), meaning "down", is an ion with fewer electrons than protons, giving it a positive charge. The suffixes for cationic groups discern between 'ylium' for cations created by the loss of a hydride ion and 'ium' for cations formed by addition of a Hydron. Methods of cationization Cationization involves the modification of cellulosic macromolecules with positively charged sites with chemical reaction of cationic reactive agents for example with Quaternary ammonium cation or using (3-chloro-2-hydroxylpropyl) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Charge
Surface charge is a two-dimensional surface with non-zero electric charge. These electric charges are constrained on this 2-D surface, and surface charge density, measured in coulombs per square meter (C•m−2), is used to describe the charge distribution on the surface. The electric potential is continuous across a surface charge and the electric field is discontinuous, but not infinite; this is unless the surface charge consists of a dipole layer. In comparison, the potential and electric field both diverge at any point charge or linear charge. In physics, at equilibrium, an ideal conductor has no charge on its interior; instead, the entirety of the charge of the conductor resides on the surface. However, this only applies to the ideal case of infinite electrical conductivity; The majority of the charge of an actual conductor resides within the skin depth of the conductor's surface. For dielectric materials, upon the application of an external electric field, the positive charg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Onsager Reciprocal Relations
In thermodynamics, the Onsager reciprocal relations express the equality of certain ratios between flows and forces in thermodynamic systems out of equilibrium, but where a notion of local equilibrium exists. "Reciprocal relations" occur between different pairs of forces and flows in a variety of physical systems. For example, consider fluid systems described in terms of temperature, matter density, and pressure. In this class of systems, it is known that temperature differences lead to heat flows from the warmer to the colder parts of the system; similarly, pressure differences will lead to matter flow from high-pressure to low-pressure regions. What is remarkable is the observation that, when both pressure and temperature vary, temperature differences at constant pressure can cause matter flow (as in convection) and pressure differences at constant temperature can cause heat flow. Perhaps surprisingly, the heat flow per unit of pressure difference and the density (matter) flo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotachophoresis
Isotachophoresis (ITP) is a technique in analytical chemistry used for selective separation and concentration of ionic analytes. It is a form of electrophoresis; charged analytes are separated based on ionic mobility, a quantity which tells how fast an ion migrates through an electric field. Overview In conventional ITP separations, a discontinuous buffer system is used. The sample is introduced between a zone of fast leading electrolyte (LE) and a zone of slow terminating (or: trailing) electrolyte (TE). Usually, the LE and the TE have a common counterion, but the co-ions (having charges with the same sign as the analytes of interest) are different: the LE is defined by co-ions with high ionic mobility, while the TE is defined by co-ions with low ionic mobility. The analytes of interest have intermediate ionic mobility. Application of an electric potential results in a low electrical field in the leading electrolyte and a high electrical field in the terminating electrolyte. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interface And Colloid Science
Interface and colloid science is an interdisciplinary intersection of branches of chemistry, physics, nanoscience and other fields dealing with colloids, heterogeneous systems consisting of a mechanical mixture of particles between 1 nm and 1000 nm dispersed in a continuous medium. A colloidal solution is a heterogeneous mixture in which the particle size of the substance is intermediate between a true solution and a suspension, i.e. between 1–1000 nm. Smoke from a fire is an example of a colloidal system in which tiny particles of solid float in air. Just like true solutions, colloidal particles are small and cannot be seen by the naked eye. They easily pass through filter paper. But colloidal particles are big enough to be blocked by parchment paper or animal membrane. Interface and colloid science has applications and ramifications in the chemical industry, pharmaceuticals, biotechnology, ceramics, minerals, nanotechnology, and microfluidics, among others. There ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultrasound
Ultrasound is sound waves with frequency, frequencies higher than the upper audible limit of human hearing range, hearing. Ultrasound is not different from "normal" (audible) sound in its physical properties, except that humans cannot hear it. This limit varies from person to person and is approximately 20 Hertz, kilohertz (20,000 hertz) in healthy young adults. Ultrasound devices operate with frequencies from 20 kHz up to several gigahertz. Ultrasound is used in many different fields. Ultrasonic devices are used to detect objects and measure distances. Ultrasound imaging or sonography is often used in medicine. In the nondestructive testing of products and structures, ultrasound is used to detect invisible flaws. Industrially, ultrasound is used for cleaning, mixing, and accelerating chemical processes. Animals such as bats and porpoises use ultrasound for locating Predation, prey and obstacles. History Acoustics, the science of sound, starts as far back as Pyth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloid Vibration Current
Colloid vibration current is an electroacoustic phenomenon that arises when ultrasound propagates through a fluid that contains ions and either solid particles or emulsion droplets. Dukhin, A.S. and Goetz, P.J"Characterization of liquids, nano- and micro- particulates and porous bodies using Ultrasound" Elsevier, 2017 The pressure gradient in an ultrasonic wave moves particles relative to the fluid. This motion disturbs the double layer that exists at the particle-fluid interface. The picture illustrates the mechanism of this distortion. Practically all particles in fluids carry a surface charge. This surface charge is screened with an equally charged diffuse layer; this structure is called the double layer. Ions of the diffuse layer are located in the fluid and can move with the fluid. Fluid motion relative to the particle drags these diffuse ions in the direction of one or the other of the particle's poles. The picture shows ions dragged towards the left hand pole. As a resu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streaming Potential/current
Streaming media is multimedia that is delivered and consumed in a continuous manner from a source, with little or no intermediate storage in network elements. ''Streaming'' refers to the delivery method of content, rather than the content itself. Distinguishing delivery method from the media applies specifically to telecommunications networks, as most of the traditional media delivery systems are either inherently ''streaming'' (e.g. radio, television) or inherently ''non-streaming'' (e.g. books, videotape, audio CDs). There are challenges with streaming content on the Internet. For example, users whose Internet connection lacks sufficient bandwidth may experience stops, lags, or poor buffering of the content, and users lacking compatible hardware or software systems may be unable to stream certain content. With the use of buffering of the content for just a few seconds in advance of playback, the quality can be much improved. Livestreaming is the real-time delivery of cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word ''suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study was introduced in 1845 by Itali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |