Sp3 bond on:
[Wikipedia]
[Google]
[Amazon]
In
 Hybridisation describes the bonding of atoms from an atom's point of view. For a tetrahedrally coordinated carbon (e.g.,
Hybridisation describes the bonding of atoms from an atom's point of view. For a tetrahedrally coordinated carbon (e.g.,  translates into :
translates into :


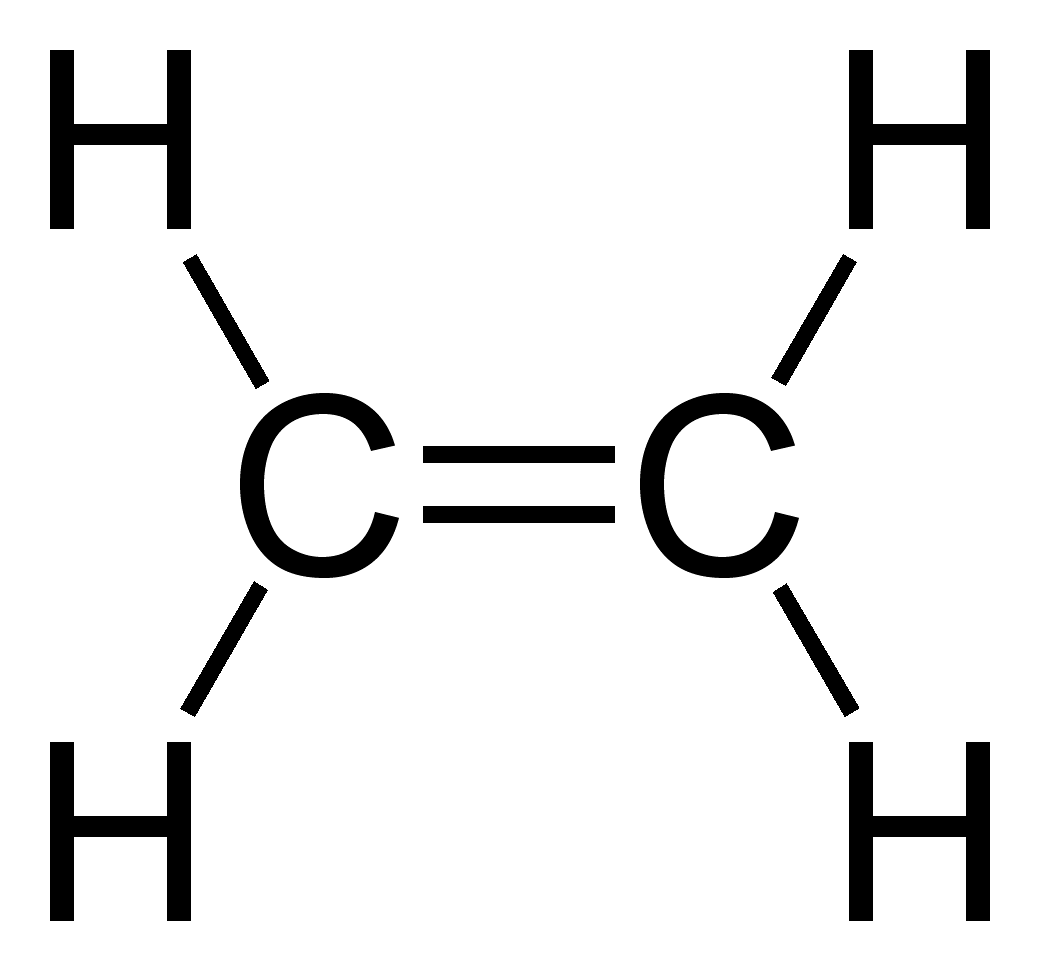 Other carbon compounds and other molecules may be explained in a similar way. For example,
Other carbon compounds and other molecules may be explained in a similar way. For example,
 The chemical bonding in compounds such as
The chemical bonding in compounds such as
 Hybridisation helps to explain molecule shape, since the angles between bonds are approximately equal to the angles between hybrid orbitals. This is in contrast to valence shell electron-pair repulsion (VSEPR) theory, which can be used to predict molecular geometry based on empirical rules rather than on valence-bond or orbital theories.
Hybridisation helps to explain molecule shape, since the angles between bonds are approximately equal to the angles between hybrid orbitals. This is in contrast to valence shell electron-pair repulsion (VSEPR) theory, which can be used to predict molecular geometry based on empirical rules rather than on valence-bond or orbital theories.

 Molecules with multiple bonds or multiple lone pairs can have orbitals represented in terms of sigma and pi symmetry or equivalent orbitals. Different valence bond methods use either of the two representations, which have mathematically equivalent total many-electron
Molecules with multiple bonds or multiple lone pairs can have orbitals represented in terms of sigma and pi symmetry or equivalent orbitals. Different valence bond methods use either of the two representations, which have mathematically equivalent total many-electron
Covalent Bonds and Molecular Structure
Hybridisation flash movie
Hybrid orbital 3D preview program in OpenGL
Understanding Concepts: Molecular Orbitals
General Chemistry tutorial on orbital hybridization
{{authority control Chemical bonding Molecular geometry Stereochemistry Quantum chemistry
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, orbital hybridisation (or hybridization) is the concept of mixing atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
s to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s in valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that det ...
and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies.
History and uses
Chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of ...
Linus Pauling
Linus Carl Pauling ( ; February 28, 1901August 19, 1994) was an American chemist and peace activist. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 gre ...
first developed the hybridisation theory in 1931 to explain the structure of simple molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
(CH4) using atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
s. Pauling pointed out that a carbon atom forms four bonds by using one s and three p orbitals, so that "it might be inferred" that a carbon atom would form three bonds at right angles (using p orbitals) and a fourth weaker bond using the s orbital in some arbitrary direction. In reality, methane has four C–H bonds of equivalent strength. The angle between any two bonds is the tetrahedral bond angle of 109°28' (around 109.5°). Pauling supposed that in the presence of four hydrogen atoms, the s and p orbitals form four equivalent combinations which he called ''hybrid'' orbitals. Each hybrid is denoted sp3 to indicate its composition, and is directed along one of the four C–H bonds. This concept was developed for such simple chemical systems, but the approach was later applied more widely, and today it is considered an effective heuristic
A heuristic or heuristic technique (''problem solving'', '' mental shortcut'', ''rule of thumb'') is any approach to problem solving that employs a pragmatic method that is not fully optimized, perfected, or rationalized, but is nevertheless ...
for rationalizing the structures of organic compounds
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
. It gives a simple orbital picture equivalent to Lewis structures.
Hybridisation theory is an integral part of organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, one of the most compelling examples being Baldwin's rules. For drawing reaction mechanisms sometimes a classical bonding picture is needed with two atoms sharing two electrons. Hybridisation theory explains bonding in alkenes and methane. The amount of p character or s character, which is decided mainly by orbital hybridisation, can be used to reliably predict molecular properties such as acidity or basicity.
Overview
Orbitals are a model representation of the behavior of electrons within molecules. In the case of simple hybridization, this approximation is based onatomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
s, similar to those obtained for the hydrogen atom, the only neutral atom for which the Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
can be solved exactly. In heavier atoms, such as carbon, nitrogen, and oxygen, the atomic orbitals used are the 2s and 2p orbitals, similar to excited state orbitals for hydrogen.
Hybrid orbitals are assumed to be mixtures of atomic orbitals, superimposed on each other in various proportions. For example, in methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, the C hybrid orbital which forms each carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
–hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
bond consists of 25% s character and 75% p character and is thus described as sp3 (read as ''s-p-three'') hybridised. Quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
describes this hybrid as an sp3 wavefunction
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter) ...
of the form , where N is a normalisation constant (here 1/2) and pσ is a p orbital directed along the C-H axis to form a sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
. The ratio of coefficients (denoted λ in general) is in this example. Since the electron density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
associated with an orbital is proportional to the square of the wavefunction, the ratio of p-character to s-character is λ2 = 3. The p character or the weight of the p component is N2λ2 = 3/4.
Types of hybridisation
sp3
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
CH4), the carbon should have 4 orbitals directed towards the 4 hydrogen atoms.
Carbon's ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state ...
configuration is 1s2 2s2 2p2 or more easily read:
This diagram suggests that the carbon atom could use its two singly occupied p-type orbitals to form two covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s with two hydrogen atoms in a methylene (CH2) molecule, with a hypothetical bond angle of 90° corresponding to the angle between two p orbitals on the same atom. However the true H–C–H angle in singlet methylene is about 102°Isaiah Shavitt (1985), ''Geometry and singlet-triplet energy gap in methylene: A critical review of experimental and theoretical determinations''. Tetrahedron, volume 41, issue 8, page 1531 which implies the presence of some orbital hybridisation.
The carbon atom can also bond to four hydrogen atoms in methane by an excitation (or promotion) of an electron from the doubly occupied 2s orbital to the empty 2p orbital, producing four singly occupied orbitals.
The energy released by the formation of two additional bonds more than compensates for the excitation energy required, energetically favoring the formation of four C-H bonds.
According to quantum mechanics, the lowest energy is obtained if the four bonds are equivalent, which requires that they are formed from equivalent orbitals on the carbon. A set of four equivalent orbitals can be obtained that are linear combinations of the valence-shell (core orbitals are almost never involved in bonding) s and p wave functions, which are the four sp3 hybrids.
In CH4, four sp3 hybrid orbitals are overlapped by the four hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
s' 1s orbitals, yielding four σ (sigma) bonds (that is, four single covalent bonds) of equal length and strength.
The following :
sp2
 Other carbon compounds and other molecules may be explained in a similar way. For example,
Other carbon compounds and other molecules may be explained in a similar way. For example, ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
(C2H4) has a double bond between the carbons. For this molecule, carbon sp2 hybridises, because one π (pi) bond is required for the double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
between the carbons and only three σ bonds are formed per carbon atom. In sp2 hybridisation the 2s orbital is mixed with only two of the three available 2p orbitals, usually denoted 2px and 2py. The third 2p orbital (2pz) remains unhybridised.
forming a total of three sp2 orbitals with one remaining p orbital. In ethylene, the two carbon atoms form a σ bond by overlapping one sp2 orbital from each carbon atom. The π bond between the carbon atoms perpendicular to the molecular plane is formed by 2p–2p overlap. Each carbon atom forms covalent C–H bonds with two hydrogens by s–sp2 overlap, all with 120° bond angles. The hydrogen–carbon bonds are all of equal strength and length, in agreement with experimental data.
sp
alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s with triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
s is explained by sp hybridization. In this model, the 2s orbital is mixed with only one of the three p orbitals,
resulting in two sp orbitals and two remaining p orbitals. The chemical bonding in acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
(ethyne) (C2H2) consists of sp–sp overlap between the two carbon atoms forming a σ bond and two additional π bonds formed by p–p overlap. Each carbon also bonds to hydrogen in a σ s–sp overlap at 180° angles.
Hybridisation and molecule shape
spx hybridisation
As the valence orbitals ofmain group element
In chemistry and atomic physics, the main group is the group (periodic table), group of chemical element, elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon ...
s are the one s and three p orbitals with the corresponding octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
, spx hybridization is used to model the shape of these molecules.
spxdy hybridisation
As the valence orbitals oftransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s are the five d, one s and three p orbitals with the corresponding 18-electron rule, spxdy hybridisation is used to model the shape of these molecules. These molecules tend to have multiple shapes corresponding to the same hybridization due to the different d-orbitals involved. A square planar complex has one unoccupied p-orbital and hence has 16 valence electrons.
sdx hybridisation
In certaintransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
complexes with a low d electron count
The d electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The d electron count is an effective way to unders ...
, the p-orbitals are unoccupied and sdx hybridisation is used to model the shape of these molecules.
Hybridisation of hypervalent molecules
Octet expansion
In some general chemistry textbooks, hybridization is presented for main group coordination number 5 and above using an "expanded octet" scheme with d-orbitals first proposed by Pauling. However, such a scheme is now considered to be incorrect in light of computational chemistry calculations. In 1990, Eric Alfred Magnusson of theUniversity of New South Wales
The University of New South Wales (UNSW) is a public research university based in Sydney, New South Wales, Australia. It was established in 1949.
The university comprises seven faculties, through which it offers bachelor's, master's and docto ...
published a paper definitively excluding the role of d-orbital hybridisation in bonding in hypervalent compounds of second-row ( period 3) elements, ending a point of contention and confusion. Part of the confusion originates from the fact that d-functions are essential in the basis sets used to describe these compounds (or else unreasonably high energies and distorted geometries result). Also, the contribution of the d-function to the molecular wavefunction is large. These facts were incorrectly interpreted to mean that d-orbitals must be involved in bonding.
Resonance
In light ofcomputational chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of mol ...
, a better treatment would be to invoke sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
in addition to hybridisation, which implies that each resonance structure has its own hybridisation scheme. All resonance structures must obey the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
.
Hybridisation in computational VB theory
While the simple model of orbital hybridisation is commonly used to explain molecular shape, hybridisation is used differently when computed in modern valence bond programs. Specifically, hybridisation is not determined ''a priori'' but is instead variationally optimized to find the lowest energy solution and then reported. This means that all artificial constraints, specifically two constraints, on orbital hybridisation are lifted: * that hybridisation is restricted to integer values (isovalent hybridisation) * that hybrid orbitals are orthogonal to one another (hybridisation defects) This means that in practice, hybrid orbitals do not conform to the simple ideas commonly taught and thus in scientific computational papers are simply referred to as spx, spxdy or sdx hybrids to express their nature instead of more specific integer values.Isovalent hybridisation
Although ideal hybrid orbitals can be useful, in reality, most bonds require orbitals of intermediate character. This requires an extension to include flexible weightings of atomic orbitals of each type (s, p, d) and allows for a quantitative depiction of the bond formation when the molecular geometry deviates from ideal bond angles. The amount of p-character is not restricted to integer values; i.e., hybridizations like sp2.5 are also readily described. The hybridization of bond orbitals is determined by Bent's rule: "Atomic s character concentrates in orbitals directed towards electropositive substituents". For molecules with lone pairs, the bonding orbitals are isovalent spx hybrids. For example, the two bond-forming hybrid orbitals of oxygen in water can be described as sp4.0 to give the interorbital angle of 104.5°. This means that they have 20% s character and 80% p character and does ''not'' imply that a hybrid orbital is formed from one s and four p orbitals on oxygen since the 2p subshell of oxygen only contains three p orbitals.Hybridisation defects
Hybridisation of s and p orbitals to form effective spx hybrids requires that they have comparable radial extent. While 2p orbitals are on average less than 10% larger than 2s, in part attributable to the lack of a radial node in 2p orbitals, 3p orbitals which have one radial node, exceed the 3s orbitals by 20–33%. The difference in extent of s and p orbitals increases further down a group. The hybridisation of atoms in chemical bonds can be analysed by considering localised molecular orbitals, for example using natural localised molecular orbitals in anatural bond orbital
In quantum chemistry, a natural bond orbital or NBO is a calculated ''bonding orbital'' with maximum electron density. The NBOs are one of a sequence of natural localized orbital sets that include "natural atomic orbitals" (NAO), "natural hybrid o ...
(NBO) scheme. In methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, CH4, the calculated p/s ratio is approximately 3 consistent with "ideal" sp3 hybridisation, whereas for silane
Silane (Silicane) is an inorganic compound with chemical formula . It is a colorless, pyrophoric gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental ...
, SiH4, the p/s ratio is closer to 2. A similar trend is seen for the other 2p elements. Substitution of fluorine for hydrogen further decreases the p/s ratio. The 2p elements exhibit near ideal hybridisation with orthogonal hybrid orbitals. For heavier p block elements this assumption of orthogonality cannot be justified. These deviations from the ideal hybridisation were termed hybridisation defects by Kutzelnigg.
However, computational VB groups such as Gerratt, Cooper and Raimondi (SCVB) as well as Shaik and Hiberty (VBSCF) go a step further to argue that even for model molecules such as methane, ethylene and acetylene, the hybrid orbitals are already defective and nonorthogonal, with hybridisations such as sp1.76 instead of sp3 for methane.
Photoelectron spectra
One misconception concerning orbital hybridization is that it incorrectly predicts the ultraviolet photoelectron spectra of many molecules. While this is true ifKoopmans' theorem
Koopmans' theorem states that in closed-shell Hartree–Fock theory (HF), the first ionization energy of a molecular system is equal to the negative of the orbital energy of the highest occupied molecular orbital (HOMO). This theorem is named afte ...
is applied to localized hybrids, quantum mechanics requires that the (in this case ionized) wavefunction obey the symmetry of the molecule which implies resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
in valence bond theory. For example, in methane, the ionised states (CH4+) can be constructed out of four resonance structures attributing the ejected electron to each of the four sp3 orbitals. A linear combination of these four structures, conserving the number of structures, leads to a triply degenerate T2 state and an A1 state. The difference in energy between each ionized state and the ground state would be ionization energy
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, Ion, positive ion, or molecule. The first ionization energy is quantitatively expressed as
: ...
, which yields two values in agreement with experimental results.

Localized vs canonical molecular orbitals
Bonding orbitals formed from hybrid atomic orbitals may be considered as localized molecular orbitals, which can be formed from the delocalized orbitals of molecular orbital theory by an appropriate mathematical transformation. For molecules in the ground state, this transformation of the orbitals leaves the total many-electron wave function unchanged. The hybrid orbital description of the ground state is, therefore ''equivalent'' to the delocalized orbital description for ground state total energy and electron density, as well as the molecular geometry that corresponds to the minimum total energy value.Two localized representations
 Molecules with multiple bonds or multiple lone pairs can have orbitals represented in terms of sigma and pi symmetry or equivalent orbitals. Different valence bond methods use either of the two representations, which have mathematically equivalent total many-electron
Molecules with multiple bonds or multiple lone pairs can have orbitals represented in terms of sigma and pi symmetry or equivalent orbitals. Different valence bond methods use either of the two representations, which have mathematically equivalent total many-electron wave function
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter) ...
s and are related by a unitary transformation
In mathematics, a unitary transformation is a linear isomorphism that preserves the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation.
Formal definition
More precise ...
of the set of occupied molecular orbitals.
For multiple bonds, the sigma-pi representation is the predominant one compared to the equivalent orbital ( bent bond) representation. In contrast, for multiple lone pairs, most textbooks use the equivalent orbital representation. However, the sigma-pi representation is also used, such as by Weinhold and Landis within the context of natural bond orbital
In quantum chemistry, a natural bond orbital or NBO is a calculated ''bonding orbital'' with maximum electron density. The NBOs are one of a sequence of natural localized orbital sets that include "natural atomic orbitals" (NAO), "natural hybrid o ...
s, a localized orbital theory containing modernized analogs of classical (valence bond/Lewis structure) bonding pairs and lone pairs. For the hydrogen fluoride molecule, for example, two F lone pairs are essentially unhybridized p orbitals, while the other is an sp''x'' hybrid orbital. An analogous consideration applies to water (one O lone pair is in a pure p orbital, another is in an sp''x'' hybrid orbital).
See also
* Crystal field theory * Isovalent hybridisation * Ligand field theory *Linear combination of atomic orbitals
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunc ...
* MO diagrams
* VALBOND
References
External links
Covalent Bonds and Molecular Structure
Hybridisation flash movie
Hybrid orbital 3D preview program in OpenGL
Understanding Concepts: Molecular Orbitals
General Chemistry tutorial on orbital hybridization
{{authority control Chemical bonding Molecular geometry Stereochemistry Quantum chemistry