|
Isovalent Hybridization
In chemistry, isovalent or second order hybridization is an extension of orbital hybridization, the mixing of atomic orbitals into hybrid orbitals which can form chemical bonds, to include fractional numbers of atomic orbitals of each type (s, p, d). It allows for a quantitative depiction of bond formation when the molecular geometry deviates from ideal bond angles. Only bonding with 4 equivalent substituents results in exactly hybridization. For molecules with different substituents, we can use isovalent hybridization to rationalize the differences in bond angles between different atoms. In the molecule methyl fluoride for example, the HCF bond angle (108.73°) is less than the HCH bond angle (110.2°). This difference can be attributed to more character in the C−F bonding and more character in the C−H bonding orbitals. The hybridisation of bond orbitals is determined by Bent's rule: "Atomic s character concentrates in orbitals directed toward electropositive substituents". ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work (pharmacology), and how to collect DNA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolate potassium metal. By heating potassium carbonate with carbon at very high temperatures, he pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bent Bond
In organic chemistry, a bent bond, also known as a banana bond, is a type of covalent chemical bond with a geometry somewhat reminiscent of a banana. The term itself is a general representation of electron density or configuration resembling a similar "bent" structure within small ring molecules, such as cyclopropane (C3H6) or as a representation of double or triple bonds within a compound that is an alternative to the sigma and pi bond model. Small cyclic molecules Bent bonds are a special type of chemical bonding in which the ordinary hybridization state of two atoms making up a chemical bond are modified with increased or decreased s-orbital character in order to accommodate a particular molecular geometry. Bent bonds are found in strained organic compounds such as cyclopropane, oxirane and aziridine. In these compounds, it is not possible for the carbon atoms to assume the 109.5° bond angles with standard sp3 hybridization. Increasing the p-character to sp5 (i.e. s-den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains are classified as small ( cyclopropane and cyclobutane), common ( cyclopentane, cyclohexane, and cycloheptane), medium ( cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons that are polycyclic. In any case, the general form of the chemical formula for cycloalkanes is C'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMR Spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. The principle of NMR usually involves three sequential steps: # The alignment (polarization) of the magnetic nuclear spins in an applied, constant magnetic field B0. # The p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
J-coupling
In nuclear chemistry and nuclear physics, ''J''-couplings (also called spin-spin coupling or indirect dipole–dipole coupling) are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that arises from hyperfine interactions between the nuclei and local electrons. In NMR spectroscopy, ''J''-coupling contains information about relative bond distances and angles. Most importantly, ''J''-coupling provides information on the connectivity of chemical bonds. It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules. ''J''-coupling is a frequency ''difference'' that is not affected by the strength of the magnetic field, so is always stated in Hz. Vector model and manifestations for chemical structure assignments The origin of ''J''-coupling can be visualized by a vector model for a simple molecule such as hydrogen fluoride (HF). In HF, the two nuclei have spin . Four state ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles Coulson
Charles Alfred Coulson (13 December 1910 – 7 January 1974) was a British applied mathematician and theoretical chemist. Coulson's major scientific work was as a pioneer of the application of the quantum theory of valency to problems of molecular structure, dynamics and reactivity. He was also a Methodist lay preacher, served on the World Council of Churches from 1962 to 1968, and was chairman of Oxfam from 1965 to 1971. Early life The parents of Charles Coulson and his younger twin brother John Metcalfe Coulson were educators who hailed from the Midlands. The twins were born when their father, Alfred, was principal of Dudley Technical College and superintendent of the Methodist Sunday School, and their mother Annie Sincere Hancock was Headmistress of Tipton Elementary School, close by. Coulson's parents maintained a religious Methodist home. When the Coulson brothers were 10, their father was appointed Superintendent of Technical Colleges for the South-West of England ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mislow, K
Kurt Martin Mislow (June 5, 1923 – October 5, 2017) was a German-born American organic chemist who specialized in stereochemistry. Born in Berlin on June 5, 1923, Mislow had moved to London by 1938, after some time in Milan. With the help of his uncle Alfred Eisenstaedt, Mislow's family left London for New York City in 1940. Mislow earned a bachelor's degree in chemistry from Tulane University in 1944, and received a doctorate from the California Institute of Technology, where he was supervised by Linus Pauling. Mislow first taught at New York University, then moved to Princeton University in 1964. While at Princeton, Mislow served as Hugh Stott Taylor Professor of Chemistry and led the chemistry department from 1968 to 1974. He became a professor emeritus in 1988. Over the course of his career, Mislow was named a Guggenheim fellow twice, in 1956 and 1974. Between 1959 and 1963, Mislow was granted the Sloan Research Fellowship. He became a member of the National Academy of Scie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene
Ethylene ( IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds). Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic compound. Much of this production goes toward polyethylene, a widely used plastic containing polymer chains of ethylene units in various chain lengths. Ethylene is also an important natural plant hormone and is used in agriculture to force the ripening of fruits. The hydrate of ethylene is ethanol. Structure and properties This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon. The molecule is also relatively weak: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
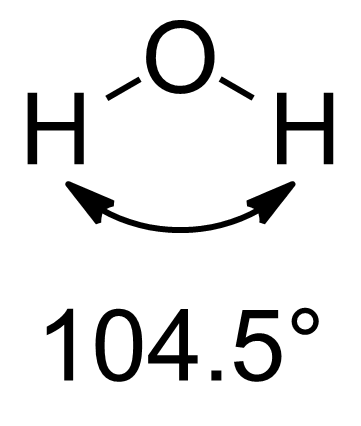
Orbital Hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production. Related compounds may be formed by replacing a hydrogen atom with another functional group; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol, the alcohol in beverages. History Ethane was first synthesised in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further. During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe and Edward Frankland produced ethane by the reductions of propionitrile ( e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bent's Rule
In chemistry, Bent's rule describes and explains the relationship between the orbital hybridization of central atoms in molecules and the electronegativities of substituents. The rule was stated by Henry A. Bent as follows: The chemical structure of a molecule is intimately related to its properties and reactivity. Valence bond theory proposes that molecular structures are due to covalent bonds between the atoms and that each bond consists of two overlapping and typically hybridised atomic orbitals. Traditionally, p-block elements in molecules are assumed to hybridise strictly as sp''n'', where ''n'' is either 1, 2, or 3. In addition, the hybrid orbitals are all assumed to be equivalent (i.e. the sp''n'' orbitals have the same p character). Results from this approach are usually good, but they can be improved upon by allowing isovalent hybridization, in which the hybridised orbitals may have noninteger and unequal p character. Bent's rule provides a qualitative estimate a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |