Single-electron Transfer on:
[Wikipedia]
[Google]
[Amazon]

 In
In
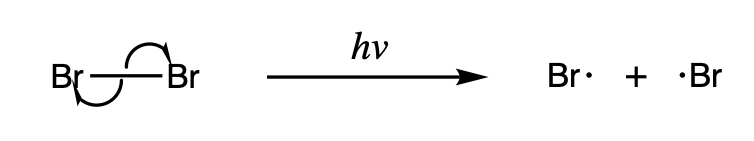
 Homolysis makes two new radicals from a spin-paired molecule by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond. Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light. The bond dissociation energy associated with homolysis depends on the stability of a given compound, and some weak bonds are able to homolyze at relatively lower temperatures.
Some homolysis reactions are particularly important because they serve as an initiator for other radical reactions. One such example is the homolysis of halogens, which occurs under light and serves as the driving force for radical halogenation reactions.
Another notable reaction is the homolysis of dibenzoyl peroxide, which results in the formation of two benzoyloxy radicals and acts as an initiator for many radical reactions.
Homolysis makes two new radicals from a spin-paired molecule by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond. Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light. The bond dissociation energy associated with homolysis depends on the stability of a given compound, and some weak bonds are able to homolyze at relatively lower temperatures.
Some homolysis reactions are particularly important because they serve as an initiator for other radical reactions. One such example is the homolysis of halogens, which occurs under light and serves as the driving force for radical halogenation reactions.
Another notable reaction is the homolysis of dibenzoyl peroxide, which results in the formation of two benzoyloxy radicals and acts as an initiator for many radical reactions.


 Hydrogen abstraction describes when a hydrogen atom is removed from a hydrogen donor molecule (e.g. tin or silicon hydride) with its one electron. Abstraction produces a new radical and a new spin-paired molecule. This is different from homolysis, which results in two radicals from a single spin-paired molecule and doesn’t include a radical as its reactant. Hydrogen abstraction is a fundamental process in radical chemistry because it serves as the final propagation step in many chemical reactions, converting carbon radicals into stable molecules. The figure to the right shows a radical abstraction between a benzoyloxy radical and a hydrogen bromide molecule, resulting in the production of a benzoic acid molecule and a bromine radical.
Hydrogen abstraction describes when a hydrogen atom is removed from a hydrogen donor molecule (e.g. tin or silicon hydride) with its one electron. Abstraction produces a new radical and a new spin-paired molecule. This is different from homolysis, which results in two radicals from a single spin-paired molecule and doesn’t include a radical as its reactant. Hydrogen abstraction is a fundamental process in radical chemistry because it serves as the final propagation step in many chemical reactions, converting carbon radicals into stable molecules. The figure to the right shows a radical abstraction between a benzoyloxy radical and a hydrogen bromide molecule, resulting in the production of a benzoic acid molecule and a bromine radical.

 Although organic radicals are generally stable intrinsically (in isolation), practically speaking their existence is only transient because they tend to dimerize. Some are quite long-lived. Generally organic radicals are stabilized by any or all of these factors: presence of electronegativity, delocalization, and steric hindrance. The compound 2,2,6,6-tetramethylpiperidinyloxyl illustrates the combination of all three factors. It is a commercially available solid that, aside from being magnetic, behaves like a normal organic compound.
Although organic radicals are generally stable intrinsically (in isolation), practically speaking their existence is only transient because they tend to dimerize. Some are quite long-lived. Generally organic radicals are stabilized by any or all of these factors: presence of electronegativity, delocalization, and steric hindrance. The compound 2,2,6,6-tetramethylpiperidinyloxyl illustrates the combination of all three factors. It is a commercially available solid that, aside from being magnetic, behaves like a normal organic compound.
 Delocalization effects can also be understood using molecular orbital theory as a lens, more specifically, by examining the intramolecular interaction of the unpaired electron with a donating group’s pair of electrons or the empty π* orbital of an electron-withdrawing group in the form of a molecular orbital diagram. The HOMO of a radical is singly-occupied hence the orbital is aptly referred to as the SOMO, or the Singly-Occupied Molecular Orbital. For an electron-donating group, the SOMO interacts with the lower energy lone pair to form a new lower-energy filled bonding-orbital and a singly-filled new SOMO, higher in energy than the original. While the energy of the unpaired electron has increased, the decrease in energy of the lone pair forming the new bonding orbital outweighs the increase in energy of the new SOMO, resulting in a net decrease of the energy of the molecule. Therefore, electron-donating groups help stabilize radicals.
Delocalization effects can also be understood using molecular orbital theory as a lens, more specifically, by examining the intramolecular interaction of the unpaired electron with a donating group’s pair of electrons or the empty π* orbital of an electron-withdrawing group in the form of a molecular orbital diagram. The HOMO of a radical is singly-occupied hence the orbital is aptly referred to as the SOMO, or the Singly-Occupied Molecular Orbital. For an electron-donating group, the SOMO interacts with the lower energy lone pair to form a new lower-energy filled bonding-orbital and a singly-filled new SOMO, higher in energy than the original. While the energy of the unpaired electron has increased, the decrease in energy of the lone pair forming the new bonding orbital outweighs the increase in energy of the new SOMO, resulting in a net decrease of the energy of the molecule. Therefore, electron-donating groups help stabilize radicals.
 With a group that is instead electron-withdrawing, the SOMO then interacts with the empty π* orbital. There are no electrons occupying the higher energy orbital formed, while a new SOMO forms that is lower in energy. This results in a lower energy and higher stability of the radical species. Both donating groups and withdrawing groups stabilize radicals.
With a group that is instead electron-withdrawing, the SOMO then interacts with the empty π* orbital. There are no electrons occupying the higher energy orbital formed, while a new SOMO forms that is lower in energy. This results in a lower energy and higher stability of the radical species. Both donating groups and withdrawing groups stabilize radicals.
 Another well-known albeit weaker form of delocalization is hyperconjugation. In radical chemistry, radicals are stabilized by hyperconjugation with adjacent alkyl groups. The donation of sigma (σ) C−H bonds into the partially empty radical orbitals helps to differentiate the stabilities of radicals on tertiary, secondary, and primary carbons. Tertiary carbon radicals have three σ C-H bonds that donate, secondary radicals only two, and primary radicals only one. Therefore, tertiary radicals are the most stable and primary radicals the least stable.
Another well-known albeit weaker form of delocalization is hyperconjugation. In radical chemistry, radicals are stabilized by hyperconjugation with adjacent alkyl groups. The donation of sigma (σ) C−H bonds into the partially empty radical orbitals helps to differentiate the stabilities of radicals on tertiary, secondary, and primary carbons. Tertiary carbon radicals have three σ C-H bonds that donate, secondary radicals only two, and primary radicals only one. Therefore, tertiary radicals are the most stable and primary radicals the least stable.
 Most simply, the greater the steric hindrance the more difficult it is for reactions to take place, and the radical form is favored by default. For example, compare the hydrogen-abstracted form of ''N''-hydroxypiperidine to the molecule TEMPO. TEMPO, or (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl, is too sterically hindered by the additional methyl groups to react making it stable enough to be sold commercially in its radical form. ''N''-Hydroxypiperidine, however, does not have the four methyl groups to impede the way of a reacting molecule so the structure is unstable.
Most simply, the greater the steric hindrance the more difficult it is for reactions to take place, and the radical form is favored by default. For example, compare the hydrogen-abstracted form of ''N''-hydroxypiperidine to the molecule TEMPO. TEMPO, or (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl, is too sterically hindered by the additional methyl groups to react making it stable enough to be sold commercially in its radical form. ''N''-Hydroxypiperidine, however, does not have the four methyl groups to impede the way of a reacting molecule so the structure is unstable.
 Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as
Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as
 Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a methyl group or a carboxyl, whether it was part of a larger molecule or a molecule on its own. The qualifier "free" was then needed to specify the unbound case. Following recent nomenclature revisions, a part of a larger molecule is now called a functional group or
Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a methyl group or a carboxyl, whether it was part of a larger molecule or a molecule on its own. The qualifier "free" was then needed to specify the unbound case. Following recent nomenclature revisions, a part of a larger molecule is now called a functional group or 28th International Symposium on Free Radicals
.
 The homolytic cleavage of the breaking bond is drawn with a 'fish-hook' arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow. The second electron of the breaking bond also moves to pair up with the attacking radical electron; this is not explicitly indicated in this case.
Radicals also take part in radical addition and radical substitution as
The homolytic cleavage of the breaking bond is drawn with a 'fish-hook' arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow. The second electron of the breaking bond also moves to pair up with the attacking radical electron; this is not explicitly indicated in this case.
Radicals also take part in radical addition and radical substitution as

 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
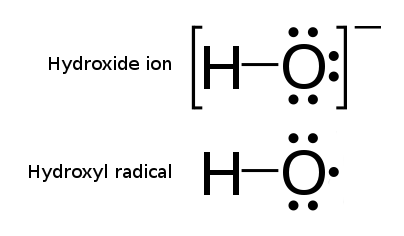
, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.
''Reactivity'' refers to:
* the chemical reactions of a single sub ...
. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons.
Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
, heat, electrical discharges, and electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations.
Radicals are important in combustion, atmospheric chemistry, polymerization, plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
chemistry, biochemistry, and many other chemical processes. A majority of natural products are generated by radical-generating enzymes. In living organisms, the radicals superoxide and nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
and their reaction products regulate many processes, such as control of vascular tone and thus blood pressure. They also play a key role in the intermediary metabolism of various biological compounds. Such radicals can even be messengers in a process dubbed redox signaling. A radical may be trapped within a solvent cage or be otherwise bound.
Formation
Radicals are either (1) formed from spin-paired molecules or (2) from other radicals. Radicals are formed from spin-paired molecules through homolysis of weak bonds or electron transfer, also known as reduction. Radicals are formed from other radicals through substitution, addition, and elimination reactions.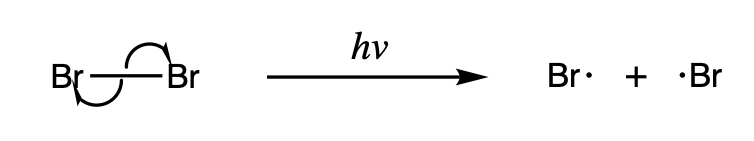
Radical formation from spin-paired molecules
Homolysis
 Homolysis makes two new radicals from a spin-paired molecule by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond. Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light. The bond dissociation energy associated with homolysis depends on the stability of a given compound, and some weak bonds are able to homolyze at relatively lower temperatures.
Some homolysis reactions are particularly important because they serve as an initiator for other radical reactions. One such example is the homolysis of halogens, which occurs under light and serves as the driving force for radical halogenation reactions.
Another notable reaction is the homolysis of dibenzoyl peroxide, which results in the formation of two benzoyloxy radicals and acts as an initiator for many radical reactions.
Homolysis makes two new radicals from a spin-paired molecule by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond. Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light. The bond dissociation energy associated with homolysis depends on the stability of a given compound, and some weak bonds are able to homolyze at relatively lower temperatures.
Some homolysis reactions are particularly important because they serve as an initiator for other radical reactions. One such example is the homolysis of halogens, which occurs under light and serves as the driving force for radical halogenation reactions.
Another notable reaction is the homolysis of dibenzoyl peroxide, which results in the formation of two benzoyloxy radicals and acts as an initiator for many radical reactions.

Reduction
Radicals can also form when a single electron is added to a spin-paired molecule, resulting in an electron transfer. This reaction, also called reduction, usually takes place with an alkali metal donating an electron to another spin-paired molecule.Radical formation from other radicals
Abstraction

 Hydrogen abstraction describes when a hydrogen atom is removed from a hydrogen donor molecule (e.g. tin or silicon hydride) with its one electron. Abstraction produces a new radical and a new spin-paired molecule. This is different from homolysis, which results in two radicals from a single spin-paired molecule and doesn’t include a radical as its reactant. Hydrogen abstraction is a fundamental process in radical chemistry because it serves as the final propagation step in many chemical reactions, converting carbon radicals into stable molecules. The figure to the right shows a radical abstraction between a benzoyloxy radical and a hydrogen bromide molecule, resulting in the production of a benzoic acid molecule and a bromine radical.
Hydrogen abstraction describes when a hydrogen atom is removed from a hydrogen donor molecule (e.g. tin or silicon hydride) with its one electron. Abstraction produces a new radical and a new spin-paired molecule. This is different from homolysis, which results in two radicals from a single spin-paired molecule and doesn’t include a radical as its reactant. Hydrogen abstraction is a fundamental process in radical chemistry because it serves as the final propagation step in many chemical reactions, converting carbon radicals into stable molecules. The figure to the right shows a radical abstraction between a benzoyloxy radical and a hydrogen bromide molecule, resulting in the production of a benzoic acid molecule and a bromine radical.
Addition
Radical addition describes when a radical is added to a spin-paired molecule to form a new radical. The figure on the right shows the addition of a bromine radical to an alkene. Radical addition follows the Anti -Markovnikov rule, where the substituent is added to the less substituted carbon atom.Elimination
Radical elimination can be viewed as the reverse of radical addition. In radical elimination, an unstable radical compound breaks down into a spin-paired molecule and a new radical compound. Shown below is an example of a radical elimination reaction, where a benzoyloxy radical breaks down into a phenyl radical and a carbon dioxide molecule.
Stability
Stability of organic radicals
Electronegativity
Organic radicals are inherently electron deficient thus the greater the electronegativity of the atom on which the unpaired electron resides the less stable the radical. Between carbon, nitrogen, and oxygen, for example, carbon is the most stable and oxygen the least stable. Electronegativity also factors into the stability of carbon atoms of different hybridizations. Greater s-character correlates to higher electronegativity of the carbon atom (due to the close proximity of s orbitals to the nucleus), and the greater the electronegativity the less stable a radical. sp-hybridized carbons (50% s-character) form the least stable radicals compared to sp3-hybridized carbons (25% s-character) which form the most stable radicals.Delocalization
The delocalization of electrons across the structure of a radical, also known as its ability to form one or more resonance structures, allows for the electron-deficiency to be spread over several atoms, minimizing instability. Delocalization usually occurs in the presence of electron-donating groups, such as hydroxyl groups (−OH), ethers (−OR), adjacent alkenes, and amines (−NH2 or −NR), or electron-withdrawing groups, such as C=O or C≡N.Steric hindrance
Facile H-atom donors
The stability of many (or most) organic radicals is not indicated by their isolability but is manifested in their ability to function as donors of H•. This property reflects a weakened bond to hydrogen, usually O−H but sometimes N−H or C−H. This behavior is important because these H• donors serve as antioxidants in biology and in commerce. Illustrative isα-tocopherol
α-Tocopherol is a type of vitamin E. It has E number "E307". Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free ra ...
( vitamin E). The tocopherol radical itself is insufficiently stable for isolation, but the parent molecule is a highly effective hydrogen-atom donor. The C−H bond is weakened in triphenylmethyl
The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trit ...
(trityl) derivatives.
upright=1.1, 2,2,6,6-Tetramethylpiperidinyloxyl is an example of a robust organic radical.
Inorganic radicals
A large variety of inorganic radicals are stable and in fact isolable. Examples include most first-row transition metal complexes. With regard to main group radicals, the most abundant radical in the universe is also the most abundant chemical in the universe, H•. Most main group radicals are not however ''isolable'', despite their intrinsic stability. Hydrogen radicals for example combine eagerly to form H2.Nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
(NO) is well known example of an isolable inorganic radical. Fremy's salt (Potassium nitrosodisulfonate, (KSO3)2NO) is a related example. Many thiazyl radicals are known, despite limited extent of π resonance stabilization.
Many radicals can be envisioned as the products of breaking of covalent bonds by homolysis. The homolytic bond dissociation energies
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
, usually abbreviated as "Δ''H''°" are a measure of bond strength. Splitting H2 into 2 H•, for example, requires a Δ''H''° of +435 kJ/mol
The joule per mole (symbol: J·mol−1 or J/mol) is the unit of energy per amount of substance in the International System of Units (SI), such that energy is measured in joules, and the amount of substance is measured in moles.
It is also an SI ...
, while splitting Cl2 into two Cl• requires a Δ''H''° of +243 kJ/mol. For weak bonds, homolysis can be induced thermally. Strong bonds require high energy photons or even flames to induce homolysis.
Diradicals
Diradicals are molecules containing two radical centers. Dioxygen (O2) is an important example of a stable diradical.Singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
, the lowest-energy non-radical state of dioxygen, is less stable than the diradical due to Hund's rule of maximum multiplicity
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configurati ...
. The relative stability of the oxygen diradical is primarily due to the spin-forbidden nature of the triplet-singlet transition required for it to grab electrons, i.e., "oxidize". The diradical state of oxygen also results in its paramagnetic character, which is demonstrated by its attraction to an external magnet. Diradicals can also occur in metal-oxo complexes, lending themselves for studies of spin forbidden reactions
In chemistry, the selection rule (also known as the transition rule) formally restricts certain reactions, known as spin-forbidden reactions, from occurring due to a required change between two differing quantum states. When a reactant exists in o ...
in transition metal chemistry. Carbenes in their triplet state can be viewed as diradicals centred on the same atom, while these are usually highly reactive persistent carbenes are known, with N-heterocyclic carbenes being the most common example.
Triplet carbenes and nitrenes are diradicals. Their chemical properties are distinct from the properties of their singlet analogues.
Occurrence of radicals
Combustion
A familiar radical reaction is combustion. The oxygen molecule is a stable diradical, best represented by •O–O•. Becausespins
The spins (as in having "the spins")Diane Marie Leiva. ''The Florida State University College of Education''Women's Voices on College Drinking: The First-Year College Experience"/ref> is an adverse reaction of intoxication that causes a state of v ...
of the electrons are parallel, this molecule is stable. While the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
of oxygen is this unreactive spin-unpaired (triplet
A triplet is a set of three items, which may be in a specific order, or unordered. It may refer to:
Science
* A series of three nucleotide bases forming an element of the Genetic code
* J-coupling as part of Nuclear magnetic resonance spectrosc ...
) diradical, an extremely reactive spin-paired ( singlet) state is available. For combustion to occur, the energy barrier
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
between these must be overcome. This barrier can be overcome by heat, requiring high temperatures. The triplet-singlet transition is also " forbidden". This presents an additional barrier to the reaction. It also means molecular oxygen is relatively unreactive at room temperature except in the presence of a catalytic heavy atom such as iron or copper.
Combustion consists of various radical chain reactions that the singlet radical can initiate. The flammability of a given material strongly depends on the concentration of radicals that must be obtained before initiation and propagation reactions dominate leading to combustion of the material. Once the combustible material has been consumed, termination reactions again dominate and the flame dies out. As indicated, promotion of propagation or termination reactions alters flammability. For example, because lead itself deactivates radicals in the gasoline-air mixture, tetraethyl lead was once commonly added to gasoline. This prevents the combustion from initiating in an uncontrolled manner or in unburnt residues (engine knocking
In spark ignition internal combustion engines, knocking (also knock, detonation, spark knock, pinging or pinking) occurs when combustion of some of the air/fuel mixture in the cylinder does not result from propagation of the flame front ignite ...
) or premature ignition ( preignition).
When a hydrocarbon is burned, a large number of different oxygen radicals are involved. Initially, hydroperoxyl radical (HOO•) are formed. These then react further to give organic hydroperoxide
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily b ...
s that break up into hydroxyl radicals (HO•).
Polymerization
Many polymerization reactions are initiated by radicals. Polymerization involves an initial radical adding to non-radical (usually an alkene) to give new radicals. This process is the basis of theradical chain reaction
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
* Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe an ...
. The art of polymerization entails the method by which the initiating radical is introduced. For example, methyl methacrylate
Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA ...
(MMA) can be polymerized to produce Poly(methyl methacrylate)
Poly(methyl methacrylate) (PMMA) belongs to a group of materials called engineering plastics. It is a transparent thermoplastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and brands Crylux, Plexiglas, Acrylite, ...
(PMMA - Plexiglas or Perspex) via a repeating series of radical addition steps:
upright=3.35, center, Radical intermediates in the formation of polymethacrylate (plexiglas or perspex)
Newer radical polymerization methods are known as living radical polymerization. Variants include reversible addition-fragmentation chain transfer ( RAFT) and atom transfer radical polymerization ( ATRP).
Being a prevalent radical, O2 reacts with many organic compounds to generate radicals together with the hydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have t ...
radical. Drying oils and alkyd paints harden due to radical crosslinking initiated by oxygen from the atmosphere.
Atmospheric radicals
The most common radical in the lower atmosphere is molecular dioxygen.Photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
of source molecules produces other radicals. In the lower atmosphere, important radical are produced by the photodissociation of nitrogen dioxide to an oxygen atom and nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
(see below), which plays a key role in smog
Smog, or smoke fog, is a type of intense air pollution. The word "smog" was coined in the early 20th century, and is a portmanteau of the words ''smoke'' and '' fog'' to refer to smoky fog due to its opacity, and odor. The word was then inte ...
formation—and the photodissociation of ozone to give the excited oxygen atom O(1D) (see below). The net and return reactions are also shown ( and , respectively).
In the upper atmosphere, the photodissociation of normally unreactive chlorofluorocarbons (CFCs) by solar ultraviolet radiation is an important source of radicals (see eq. 1 below). These reactions give the chlorine radical, Cl•, which catalyzes the conversion of ozone to O2, thus facilitating ozone depletion
Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone l ...
(– below).
Such reactions cause the depletion of the ozone layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in rela ...
, especially since the chlorine radical is free to engage in another reaction chain; consequently, the use of chlorofluorocarbons as refrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Ref ...
s has been restricted.
In biology
 Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as
Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as granulocyte
Granulocytes are
cells in the innate immune system characterized by the presence of specific granules in their cytoplasm. Such granules distinguish them from the various agranulocytes. All myeloblastic granulocytes are polymorphonuclear. They ha ...
s and macrophage
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer cel ...
s. Radicals are involved in cell signalling
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellula ...
processes, known as redox signaling. For example, radical attack of linoleic acid produces a series of 13-hydroxyoctadecadienoic acids and 9-hydroxyoctadecadienoic acid
9-Hydroxyoctadecadienoic acid (or 9-HODE) has been used in the literature to designate either or both of two stereoisomer metabolites of the essential fatty acid, linoleic acid: 9(''S'')-hydroxy-10(''E''),12(''Z'')-octadecadienoic acid (9(''S'')- ...
s, which may act to regulate localized tissue inflammatory and/or healing responses, pain perception, and the proliferation of malignant cells. Radical attacks on arachidonic acid and docosahexaenoic acid produce a similar but broader array of signaling products.
Radicals may also be involved in Parkinson's disease, senile and drug-induced deafness, schizophrenia, and Alzheimer's
Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens. It is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As t ...
. The classic free-radical syndrome, the iron-storage disease hemochromatosis, is typically associated with a constellation of free-radical-related symptoms including movement disorder, psychosis, skin pigmentary melanin abnormalities, deafness, arthritis, and diabetes mellitus. The free-radical theory of aging proposes that radicals underlie the aging process
Ageing ( BE) or aging ( AE) is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In ...
itself. Similarly, the process of mito hormesis suggests that repeated exposure to radicals may extend life span.
Because radicals are necessary for life, the body has a number of mechanisms to minimize radical-induced damage and to repair damage that occurs, such as the enzymes superoxide dismutase
Superoxide dismutase (SOD, ) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide () radical into ordinary molecular oxygen (O2) and hydrogen peroxide (). Superoxide is produced as a by-product of oxygen me ...
, catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting t ...
, glutathione peroxidase and glutathione reductase. In addition, antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricant ...
s play a key role in these defense mechanisms. These are often the three vitamins, vitamin A, vitamin C and vitamin E and polyphenol antioxidant
A polyphenol antioxidant is a hypothetical type of antioxidant containing a polyphenolic substructure and studied in vitro. Numbering over 4,000 distinct species mostly from plants, polyphenols may have antioxidant activity in vitro, but are unlik ...
s. Furthermore, there is good evidence indicating that bilirubin
Bilirubin (BR) (Latin for "red bile") is a red-orange compound that occurs in the normal catabolic pathway that breaks down heme in vertebrates. This catabolism is a necessary process in the body's clearance of waste products that arise from the ...
and uric acid can act as antioxidants to help neutralize certain radicals. Bilirubin comes from the breakdown of red blood cells' contents, while uric acid is a breakdown product of purines. Too much bilirubin, though, can lead to jaundice
Jaundice, also known as icterus, is a yellowish or greenish pigmentation of the skin and sclera due to high bilirubin levels. Jaundice in adults is typically a sign indicating the presence of underlying diseases involving abnormal heme meta ...
, which could eventually damage the central nervous system, while too much uric acid causes gout.
Reactive oxygen species
Reactive oxygen species or ROS are species such as superoxide, hydrogen peroxide, and hydroxyl radical, commonly associated with cell damage. ROS form as a natural by-product of the normal metabolism of oxygen and have important roles in cell signaling. Two important oxygen-centered radicals are superoxide and hydroxyl radical. They derive from molecular oxygen under reducing conditions. However, because of their reactivity, these same radicals can participate in unwanted side reactions resulting in cell damage. Excessive amounts of these radicals can lead to cell injury and death, which may contribute to many diseases such as cancer,stroke
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functionin ...
, myocardial infarction, diabetes and major disorders. Many forms of cancer are thought to be the result of reactions between radicals and DNA, potentially resulting in mutations that can adversely affect the cell cycle and potentially lead to malignancy. Some of the symptoms of aging
Ageing ( BE) or aging ( AE) is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In ...
such as atherosclerosis are also attributed to radical induced oxidation of cholesterol to 7-ketocholesterol. In addition radicals contribute to alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
-induced liver damage, perhaps more than alcohol itself. Radicals produced by cigarette
A cigarette is a narrow cylinder containing a combustible material, typically tobacco, that is rolled into thin paper for smoking. The cigarette is ignited at one end, causing it to smolder; the resulting smoke is orally inhaled via the opp ...
smoke are implicated in inactivation of alpha 1-antitrypsin
Alpha-1 antitrypsin or α1-antitrypsin (A1AT, α1AT, A1A, or AAT) is a protein belonging to the serpin superfamily. It is encoded in humans by the ''SERPINA1'' gene. A protease inhibitor, it is also known as alpha1–proteinase inhibitor (A1PI) ...
in the lung
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of t ...
. This process promotes the development of emphysema
Emphysema, or pulmonary emphysema, is a lower respiratory tract disease, characterised by air-filled spaces ( pneumatoses) in the lungs, that can vary in size and may be very large. The spaces are caused by the breakdown of the walls of the alve ...
.
Oxybenzone has been found to form radicals in sunlight, and therefore may be associated with cell damage as well. This only occurred when it was combined with other ingredients commonly found in sunscreens, like titanium oxide and octyl methoxycinnamate.
ROS attack the polyunsaturated fatty acid, linoleic acid, to form a series of 13-hydroxyoctadecadienoic acid and 9-hydroxyoctadecadienoic acid
9-Hydroxyoctadecadienoic acid (or 9-HODE) has been used in the literature to designate either or both of two stereoisomer metabolites of the essential fatty acid, linoleic acid: 9(''S'')-hydroxy-10(''E''),12(''Z'')-octadecadienoic acid (9(''S'')- ...
products that serve as signaling molecules that may trigger responses that counter the tissue injury which caused their formation. ROS attacks other polyunsaturated fatty acids, e.g. arachidonic acid
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). It is structurally related to the saturated arachidic acid found in cupuaçu butter. Its name derives from the New Latin word ''arachi ...
and docosahexaenoic acid, to produce a similar series of signaling products.
History and nomenclature
 Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a methyl group or a carboxyl, whether it was part of a larger molecule or a molecule on its own. The qualifier "free" was then needed to specify the unbound case. Following recent nomenclature revisions, a part of a larger molecule is now called a functional group or
Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a methyl group or a carboxyl, whether it was part of a larger molecule or a molecule on its own. The qualifier "free" was then needed to specify the unbound case. Following recent nomenclature revisions, a part of a larger molecule is now called a functional group or substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
, and "radical" now implies "free". However, the old nomenclature may still appear in some books.
The term radical was already in use when the now obsolete radical theory was developed. Louis-Bernard Guyton de Morveau introduced the phrase "radical" in 1785 and the phrase was employed by Antoine Lavoisier in 1789 in his Traité Élémentaire de Chimie
''Traité élémentaire de chimie'' (''Elementary Treatise on Chemistry'') is a textbook written by Antoine Lavoisier published in 1789 and translated into English by Robert Kerr in 1790 under the title ''Elements of Chemistry in a New Systemati ...
. A radical was then identified as the root base of certain acids (the Latin word "radix" meaning "root"). Historically, the term ''radical'' in radical theory was also used for bound parts of the molecule, especially when they remain unchanged in reactions. These are now called functional groups. For example, methyl alcohol was described as consisting of a methyl "radical" and a hydroxyl "radical". Neither are radicals in the modern chemical sense, as they are permanently bound to each other, and have no unpaired, reactive electrons; however, they can be observed as radicals in mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
when broken apart by irradiation with energetic electrons.
In a modern context the first organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
(carbon–containing) radical identified was the triphenylmethyl radical, (C6H5)3C•. This species was discovered by Moses Gomberg in 1900. In 1933 Morris S. Kharasch
Morris Selig Kharasch (August 24, 1895 – October 9, 1957) was a pioneering organic chemist best known for his work with free radical additions and polymerizations. He defined the peroxide effect, explaining how an anti-Markovnikov orientation c ...
and Frank Mayo Frank Mayo may refer to:
* Frank M. Mayo (1839–1896), American actor and comedian
* Frank Mayo (actor)
Frank Lorimer Mayo (June 28, 1889 – July 9, 1963) was an American actor. He appeared in 310 films between 1911 and 1949.
Biograp ...
proposed that free radicals were responsible for anti-Markovnikov addition of hydrogen bromide to allyl bromide.
In most fields of chemistry, the historical definition of radicals contends that the molecules have nonzero electron spin. However, in fields including spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
and astrochemistry, the definition is slightly different. Gerhard Herzberg, who won the Nobel prize for his research into the electron structure and geometry of radicals, suggested a looser definition of free radicals: "any transient (chemically unstable) species (atom, molecule, or ion)". The main point of his suggestion is that there are many chemically unstable molecules that have zero spin, such as C2, C3, CH2 and so on. This definition is more convenient for discussions of transient chemical processes and astrochemistry; therefore researchers in these fields prefer to use this loose definition..
Depiction in chemical reactions
In chemical equations, radicals are frequently denoted by a dot placed immediately to the right of the atomic symbol or molecular formula as follows: : Radicalreaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
s use single-headed arrows to depict the movement of single electrons:
reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s. Chain reactions involving radicals can usually be divided into three distinct processes. These are ''initiation'', ''propagation'', and ''termination''.
*''Initiation'' reactions are those that result in a net increase in the number of radicals. They may involve the formation of radicals from stable species as in Reaction 1 above or they may involve reactions of radicals with stable species to form more radicals.
*''Propagation'' reactions are those reactions involving radicals in which the total number of radicals remains the same.
*''Termination'' reactions are those reactions resulting in a net decrease in the number of radicals. Typically two radicals combine to form a more stable species, for example:
*:2 Cl• → Cl2
See also
*Electron pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper ...
* Globally Harmonized System of Classification and Labelling of Chemicals
* Hofmann–Löffler reaction
The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, i ...
;Free radical research
* ARC Centre of Excellence for Free Radical Chemistry and Biotechnology
References
{{Authority control Articles containing video clips Biological processes Biomolecules Chemical bonding Environmental chemistry Senescence