Silver Shield Foundation on:
[Wikipedia]
[Google]
[Amazon]
Silver is a chemical element with the
 Silver is similar in its physical and chemical properties to its two vertical neighbours in
Silver is similar in its physical and chemical properties to its two vertical neighbours in
 Silver and gold have rather low chemical affinities for oxygen, lower than copper, and it is therefore expected that silver oxides are thermally quite unstable. Soluble silver(I) salts precipitate dark-brown silver(I) oxide, Ag2O, upon the addition of alkali. (The hydroxide AgOH exists only in solution; otherwise it spontaneously decomposes to the oxide.) Silver(I) oxide is very easily reduced to metallic silver, and decomposes to silver and oxygen above 160 °C.Greenwood and Earnshaw, pp. 1181–82 This and other silver(I) compounds may be oxidized by the strong oxidizing agent peroxodisulfate to black AgO, a mixed silver(I,III) oxide of formula AgIAgIIIO2. Some other mixed oxides with silver in non-integral oxidation states, namely Ag2O3 and Ag3O4, are also known, as is Ag3O which behaves as a metallic conductor.
Silver and gold have rather low chemical affinities for oxygen, lower than copper, and it is therefore expected that silver oxides are thermally quite unstable. Soluble silver(I) salts precipitate dark-brown silver(I) oxide, Ag2O, upon the addition of alkali. (The hydroxide AgOH exists only in solution; otherwise it spontaneously decomposes to the oxide.) Silver(I) oxide is very easily reduced to metallic silver, and decomposes to silver and oxygen above 160 °C.Greenwood and Earnshaw, pp. 1181–82 This and other silver(I) compounds may be oxidized by the strong oxidizing agent peroxodisulfate to black AgO, a mixed silver(I,III) oxide of formula AgIAgIIIO2. Some other mixed oxides with silver in non-integral oxidation states, namely Ag2O3 and Ag3O4, are also known, as is Ag3O which behaves as a metallic conductor.
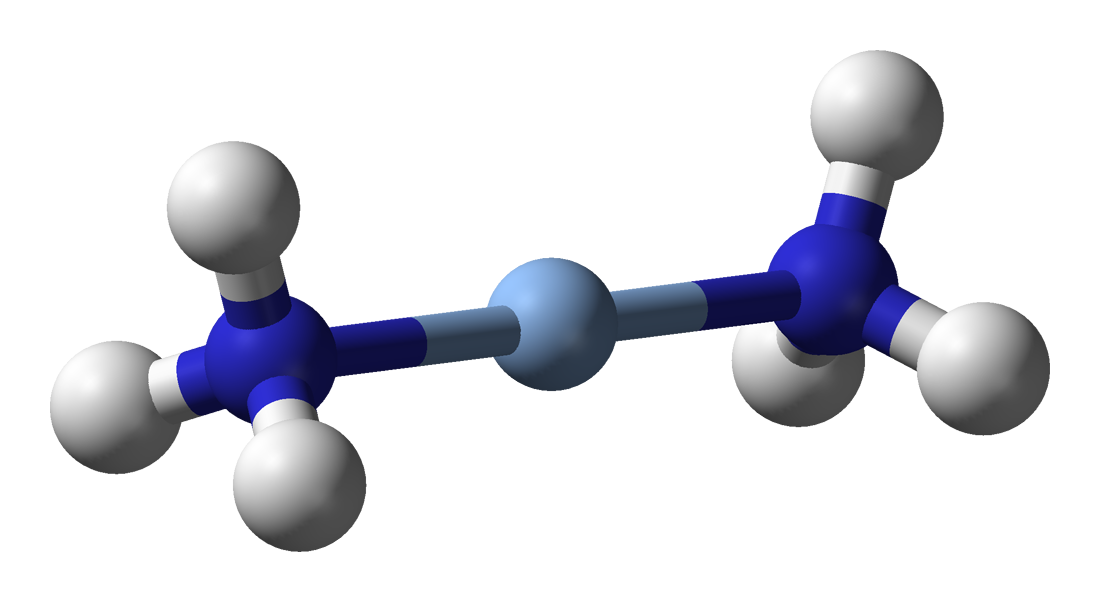 Silver complexes tend to be similar to those of its lighter homologue copper. Silver(III) complexes tend to be rare and very easily reduced to the more stable lower oxidation states, though they are slightly more stable than those of copper(III). For instance, the square planar periodate g(IO5OH)2sup>5− and tellurate g2sup>5− complexes may be prepared by oxidising silver(I) with alkaline peroxodisulfate. The yellow diamagnetic gF4sup>− is much less stable, fuming in moist air and reacting with glass.
Silver(II) complexes are more common. Like the valence isoelectronic copper(II) complexes, they are usually square planar and paramagnetic, which is increased by the greater field splitting for 4d electrons than for 3d electrons. Aqueous Ag2+, produced by oxidation of Ag+ by ozone, is a very strong oxidising agent, even in acidic solutions: it is stabilized in phosphoric acid due to complex formation. Peroxodisulfate oxidation is generally necessary to give the more stable complexes with heterocyclic amines, such as g(py)4sup>2+ and g(bipy)2sup>2+: these are stable provided the counterion cannot reduce the silver back to the +1 oxidation state. gF4sup>2− is also known in its violet barium salt, as are some silver(II) complexes with ''N''- or ''O''-donor ligands such as pyridine carboxylates.Greenwood and Earnshaw, p. 1189
By far the most important oxidation state for silver in complexes is +1. The Ag+ cation is diamagnetic, like its homologues Cu+ and Au+, as all three have closed-shell electron configurations with no unpaired electrons: its complexes are colourless provided the ligands are not too easily polarized such as I−. Ag+ forms salts with most anions, but it is reluctant to coordinate to oxygen and thus most of these salts are insoluble in water: the exceptions are the nitrate, perchlorate, and fluoride. The tetracoordinate tetrahedral aqueous ion g(H2O)4sup>+ is known, but the characteristic geometry for the Ag+ cation is 2-coordinate linear. For example, silver chloride dissolves readily in excess aqueous ammonia to form g(NH3)2sup>+; silver salts are dissolved in photography due to the formation of the thiosulfate complex g(S2O3)2sup>3−; and
Silver complexes tend to be similar to those of its lighter homologue copper. Silver(III) complexes tend to be rare and very easily reduced to the more stable lower oxidation states, though they are slightly more stable than those of copper(III). For instance, the square planar periodate g(IO5OH)2sup>5− and tellurate g2sup>5− complexes may be prepared by oxidising silver(I) with alkaline peroxodisulfate. The yellow diamagnetic gF4sup>− is much less stable, fuming in moist air and reacting with glass.
Silver(II) complexes are more common. Like the valence isoelectronic copper(II) complexes, they are usually square planar and paramagnetic, which is increased by the greater field splitting for 4d electrons than for 3d electrons. Aqueous Ag2+, produced by oxidation of Ag+ by ozone, is a very strong oxidising agent, even in acidic solutions: it is stabilized in phosphoric acid due to complex formation. Peroxodisulfate oxidation is generally necessary to give the more stable complexes with heterocyclic amines, such as g(py)4sup>2+ and g(bipy)2sup>2+: these are stable provided the counterion cannot reduce the silver back to the +1 oxidation state. gF4sup>2− is also known in its violet barium salt, as are some silver(II) complexes with ''N''- or ''O''-donor ligands such as pyridine carboxylates.Greenwood and Earnshaw, p. 1189
By far the most important oxidation state for silver in complexes is +1. The Ag+ cation is diamagnetic, like its homologues Cu+ and Au+, as all three have closed-shell electron configurations with no unpaired electrons: its complexes are colourless provided the ligands are not too easily polarized such as I−. Ag+ forms salts with most anions, but it is reluctant to coordinate to oxygen and thus most of these salts are insoluble in water: the exceptions are the nitrate, perchlorate, and fluoride. The tetracoordinate tetrahedral aqueous ion g(H2O)4sup>+ is known, but the characteristic geometry for the Ag+ cation is 2-coordinate linear. For example, silver chloride dissolves readily in excess aqueous ammonia to form g(NH3)2sup>+; silver salts are dissolved in photography due to the formation of the thiosulfate complex g(S2O3)2sup>3−; and

 Silver forms alloys with most other elements on the periodic table. The elements from groups 1–3, except for hydrogen, lithium, and beryllium, are very miscible with silver in the condensed phase and form intermetallic compounds; those from groups 4–9 are only poorly miscible; the elements in groups 10–14 (except
Silver forms alloys with most other elements on the periodic table. The elements from groups 1–3, except for hydrogen, lithium, and beryllium, are very miscible with silver in the condensed phase and form intermetallic compounds; those from groups 4–9 are only poorly miscible; the elements in groups 10–14 (except
 Silver was one of the seven
Silver was one of the seven  When the Phoenicians first came to what is now Spain, they obtained so much silver that they could not fit it all on their ships, and as a result used silver to weight their anchors instead of lead. By the time of the Greek and Roman civilizations, silver coins were a staple of the economy: the Greeks were already extracting silver from
When the Phoenicians first came to what is now Spain, they obtained so much silver that they could not fit it all on their ships, and as a result used silver to weight their anchors instead of lead. By the time of the Greek and Roman civilizations, silver coins were a staple of the economy: the Greeks were already extracting silver from
File:Proto-Elamite kneeling bull holding a spouted vessel.jpg, Proto-Elamite kneeling bull holding a spouted vessel; 3100–2900 BC; 16.3 x 6.3 x 10.8 cm; Metropolitan Museum of Art (New York City)
Horus as falcon god with Egyptian crown from the 27th dynasty (05).jpg, Ancient Egyptian figurine of
 Silver plays a certain role in mythology and has found various usage as a metaphor and in folklore. The Greek poet
Silver plays a certain role in mythology and has found various usage as a metaphor and in folklore. The Greek poet
 The earliest known coins were minted in the kingdom of
The earliest known coins were minted in the kingdom of
s.v. 'dirhem'
the karshapana from ancient India and
 Silver prices are normally quoted in Troy weight, troy ounces. One troy ounce is equal to . The London silver fix is published every working day at noon London time. This price is determined by several major international banks and is used by London bullion market members for trading that day. Prices are most commonly shown as the United States dollar (USD), the Pound sterling (GBP), and the Euro (EUR).
Silver prices are normally quoted in Troy weight, troy ounces. One troy ounce is equal to . The London silver fix is published every working day at noon London time. This price is determined by several major international banks and is used by London bullion market members for trading that day. Prices are most commonly shown as the United States dollar (USD), the Pound sterling (GBP), and the Euro (EUR).
 The major use of silver besides coinage throughout most of history was in the manufacture of jewellery and other general-use items, and this continues to be a major use today. Examples include household silver, table silver for cutlery, for which silver is highly suited due to its antibacterial properties. Western concert flutes are usually plated with or made out of sterling silver;Ullmann, pp. 65–67 in fact, most silverware is only silver-plated rather than made out of pure silver; the silver is normally put in place by
The major use of silver besides coinage throughout most of history was in the manufacture of jewellery and other general-use items, and this continues to be a major use today. Examples include household silver, table silver for cutlery, for which silver is highly suited due to its antibacterial properties. Western concert flutes are usually plated with or made out of sterling silver;Ullmann, pp. 65–67 in fact, most silverware is only silver-plated rather than made out of pure silver; the silver is normally put in place by
European Union Observatory for Nanomaterials (EUON)
silver nanoparticles are used both in pigments, as well as cosmetics.
 Pure silver metal is used as a food colouring. It has the E number, E174 designation and is approved in the European Union. Traditional Indian and Pakistani dishes sometimes include decorative silver foil known as ''vark'', and in various other cultures, silver ''dragée'' are used to decorate cakes, cookies, and other dessert items.
Photochromic lenses include silver halides, so that ultraviolet light in natural daylight liberates metallic silver, darkening the lenses. The silver halides are reformed in lower light intensities. Colourless silver chloride films are used in Particle detector, radiation detectors. Zeolite sieves incorporating Ag+ ions are used to Desalination, desalinate seawater during rescues, using silver ions to precipitate chloride as silver chloride. Silver is also used for its antibacterial properties for water sanitisation, but the application of this is limited by limits on silver consumption. Colloidal silver is similarly used to disinfect closed swimming pools; while it has the advantage of not giving off a smell like hypochlorite treatments do, colloidal silver is not effective enough for more contaminated open swimming pools. Small silver iodide crystals are used in cloud seeding to cause rain.Ullmann, pp. 83–84
The Texas Legislature designated silver the official precious metal of Texas in 2007.
Pure silver metal is used as a food colouring. It has the E number, E174 designation and is approved in the European Union. Traditional Indian and Pakistani dishes sometimes include decorative silver foil known as ''vark'', and in various other cultures, silver ''dragée'' are used to decorate cakes, cookies, and other dessert items.
Photochromic lenses include silver halides, so that ultraviolet light in natural daylight liberates metallic silver, darkening the lenses. The silver halides are reformed in lower light intensities. Colourless silver chloride films are used in Particle detector, radiation detectors. Zeolite sieves incorporating Ag+ ions are used to Desalination, desalinate seawater during rescues, using silver ions to precipitate chloride as silver chloride. Silver is also used for its antibacterial properties for water sanitisation, but the application of this is limited by limits on silver consumption. Colloidal silver is similarly used to disinfect closed swimming pools; while it has the advantage of not giving off a smell like hypochlorite treatments do, colloidal silver is not effective enough for more contaminated open swimming pools. Small silver iodide crystals are used in cloud seeding to cause rain.Ullmann, pp. 83–84
The Texas Legislature designated silver the official precious metal of Texas in 2007.
Silver
at ''The Periodic Table of Videos'' (University of Nottingham)
Society of American Silversmiths
The Silver Institute
A silver industry website
Samples of silver
Transport, Fate and Effects of Silver in the Environment
Bloomberg – Markets Precious and Industrial Metals – Silver
{{good article Silver, Chemical elements Transition metals Noble metals Precious metals Cubic minerals Minerals in space group 225 Electrical conductors Native element minerals E-number additives Chemical elements with face-centered cubic structure
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
, thermal conductivity, and reflectivity of any metal. The metal is found in the Earth's crust in the pure, free elemental form ("native silver"), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite
Chlorargyrite is the mineral form of silver chloride (AgCl). Chlorargyrite occurs as a secondary mineral phase in the oxidation of silver mineral deposits. It crystallizes in the isometric - hexoctahedral crystal class. Typically massive to colum ...
. Most silver is produced as a byproduct of copper, gold, lead, and zinc refining
{{Unreferenced, date=December 2009
Refining (also perhaps called by the mathematical term affining) is the process of purification of a (1) substance or a (2) form. The term is usually used of a natural resource that is almost in a usable form, b ...
.
Silver has long been valued as a precious metal
Precious metals are rare, naturally occurring metallic chemical elements of high economic value.
Chemically, the precious metals tend to be less reactive than most elements (see noble metal). They are usually ductile and have a high lustre. ...
. Silver metal is used in many bullion coins, sometimes alongside gold: while it is more abundant than gold, it is much less abundant as a native metal
A native metal is any metal that is found pure in its metallic form in nature. Metals that can be found as native deposits singly or in alloys include aluminium, antimony, arsenic, bismuth, cadmium, chromium, cobalt, indium, iron, manganese, m ...
. Its purity is typically measured on a per-mille basis; a 94%-pure alloy is described as "0.940 fine". As one of the seven metals of antiquity
The metals of antiquity are the seven metals which humans had identified and found use for in prehistoric times in Europe and the Middle East: gold, silver, copper, tin, lead, iron, and mercury. These seven are the metals from which the moder ...
, silver has had an enduring role in most human cultures.
Other than in currency and as an investment
Investment is the dedication of money to purchase of an asset to attain an increase in value over a period of time. Investment requires a sacrifice of some present asset, such as time, money, or effort.
In finance, the purpose of investing i ...
medium ( coins and bullion
Bullion is non-ferrous metal that has been refined to a high standard of elemental purity. The term is ordinarily applied to bulk metal used in the production of coins and especially to precious metals such as gold and silver. It comes from t ...
), silver is used in solar panels
A solar cell panel, solar electric panel, photo-voltaic (PV) module, PV panel or solar panel is an assembly of photovoltaic solar cells mounted in a (usually rectangular) frame, and a neatly organised collection of PV panels is called a phot ...
, water filtration
A water filter removes impurities by lowering contamination of water using a fine physical barrier, a chemical process, or a biological process. Filters cleanse water to different extents, for purposes such as: providing agricultural irrigation ...
, jewellery, ornaments, high-value tableware and utensils (hence the term "silverware
Silverware may refer to:
* Household silver including
**Tableware
**Cutlery
**Candlesticks
*The work of a silversmith
* Silverware is also a slang term for a collection of trophies
A trophy is a tangible, durable reminder of a specific achieveme ...
"), in electrical contacts and conductors, in specialized mirrors, window coatings, in catalysis of chemical reactions, as a colorant in stained glass
Stained glass is coloured glass as a material or works created from it. Throughout its thousand-year history, the term has been applied almost exclusively to the windows of churches and other significant religious buildings. Although tradition ...
, and in specialized confectionery. Its compounds are used in photographic and X-ray film. Dilute solutions of silver nitrate and other silver compounds are used as disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than st ...
s and microbiocides ( oligodynamic effect), added to bandages, wound-dressings, catheters, and other medical instruments.
Characteristics
 Silver is similar in its physical and chemical properties to its two vertical neighbours in
Silver is similar in its physical and chemical properties to its two vertical neighbours in group 11
Group 11, by modern IUPAC numbering, is a group of chemical elements in the periodic table, consisting of copper (Cu), silver (Ag), and gold (Au), and roentgenium (Rg), although no chemical experiments have yet been carried out to confirm that ...
of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
: copper, and gold. Its 47 electrons are arranged in the configuration
Configuration or configurations may refer to:
Computing
* Computer configuration or system configuration
* Configuration file, a software file used to configure the initial settings for a computer program
* Configurator, also known as choice board ...
rd105s1, similarly to copper ( rd104s1) and gold ( ef145d106s1); group 11 is one of the few groups in the d-block which has a completely consistent set of electron configurations. This distinctive electron configuration, with a single electron in the highest occupied s subshell over a filled d subshell, accounts for many of the singular properties of metallic silver.
Silver is a relatively soft and extremely ductile and malleable transition metal, though it is slightly less malleable than gold. Silver crystallizes in a face-centered cubic lattice with bulk coordination number 12, where only the single 5s electron is delocalized, similarly to copper and gold.Greenwood and Earnshaw, p. 1178 Unlike metals with incomplete d-shells, metallic bonds in silver are lacking a covalent character and are relatively weak. This observation explains the low hardness and high ductility of single crystals of silver.
Silver has a brilliant, white, metallic luster that can take a high polish, and which is so characteristic that the name of the metal itself has become a colour name.Greenwood and Earnshaw, p. 1177 Unlike copper and gold, the energy required to excite an electron from the filled d band to the s-p conduction band in silver is large enough (around 385 kJ/mol) that it no longer corresponds to absorption in the visible region of the spectrum, but rather in the ultraviolet; hence, silver is not a coloured metal. Protected silver has greater optical reflectivity than aluminium at all wavelengths longer than ~450 nm. At wavelengths shorter than 450 nm, silver's reflectivity is inferior to that of aluminium and drops to zero near 310 nm.
Very high electrical and thermal conductivity are common to the elements in group 11, because their single s electron is free and does not interact with the filled d subshell, as such interactions (which occur in the preceding transition metals) lower electron mobility. The thermal conductivity of silver is among the highest of all materials, although the thermal conductivity of carbon (in the diamond allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
) and superfluid helium-4
Superfluid helium-4 is the superfluid form of helium-4, an isotope of the element helium. A superfluid is a state of matter in which matter behaves like a fluid with zero viscosity. The substance, which looks like a normal liquid, flows without ...
are higher. The electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
of silver is the highest of all metals, greater even than copper. Silver also has the lowest contact resistance of any metal. Silver is rarely used for its electrical conductivity, due to its high cost, although an exception is in radio-frequency engineering, particularly at VHF
Very high frequency (VHF) is the ITU designation for the range of radio frequency electromagnetic waves (radio waves) from 30 to 300 megahertz (MHz), with corresponding wavelengths of ten meters to one meter.
Frequencies immediately below VHF ...
and higher frequencies where silver plating improves electrical conductivity because those currents tend to flow on the surface of conductors rather than through the interior. During World War II in the US, tons of silver were used for the electromagnets
An electromagnet is a type of magnet in which the magnetic field is produced by an electric current. Electromagnets usually consist of wire wound into a coil. A current through the wire creates a magnetic field which is concentrated in t ...
in calutrons for enriching uranium, mainly because of the wartime shortage of copper.
Silver readily forms alloys with copper, gold, and zinc. Zinc-silver alloys with low zinc concentration may be considered as face-centred cubic solid solutions of zinc in silver, as the structure of the silver is largely unchanged while the electron concentration rises as more zinc is added. Increasing the electron concentration further leads to body-centred cubic (electron concentration 1.5), complex cubic (1.615), and hexagonal close-packed phases (1.75).
Isotopes
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag, with 107Ag being slightly more abundant (51.839% natural abundance). This almost equal abundance is rare in the periodic table. The atomic weight is 107.8682(2) u; this value is very important because of the importance of silver compounds, particularly halides, in gravimetric analysis. Both isotopes of silver are produced in stars via thes-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynthesis) of approximat ...
(slow neutron capture), as well as in supernovas via the r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
(rapid neutron capture).
Twenty-eight radioisotopes have been characterized, the most stable being 105Ag with a half-life of 41.29 days, 111Ag with a half-life of 7.45 days, and 112Ag with a half-life of 3.13 hours. Silver has numerous nuclear isomers, the most stable being 108mAg (''t''1/2 = 418 years), 110mAg (''t''1/2 = 249.79 days) and 106mAg (''t''1/2 = 8.28 days). All of the remaining radioactive isotopes have half-lives of less than an hour, and the majority of these have half-lives of less than three minutes.
Isotopes of silver range in relative atomic mass from 92.950 u (93Ag) to 129.950 u (130Ag); the primary decay mode
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
before the most abundant stable isotope, 107Ag, is electron capture and the primary mode after is beta decay. The primary decay product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( ...
s before 107Ag are palladium (element 46) isotopes, and the primary products after are cadmium (element 48) isotopes.
The palladium isotope 107Pd decays by beta emission to 107Ag with a half-life of 6.5 million years. Iron meteorites are the only objects with a high-enough palladium-to-silver ratio to yield measurable variations in 107Ag abundance. Radiogenic 107Ag was first discovered in the Santa Clara meteorite in 1978. 107Pd–107Ag correlations observed in bodies that have clearly been melted since the accretion
Accretion may refer to:
Science
* Accretion (astrophysics), the formation of planets and other bodies by collection of material through gravity
* Accretion (meteorology), the process by which water vapor in clouds forms water droplets around nucl ...
of the Solar System must reflect the presence of unstable nuclides in the early Solar System.
Chemistry
Silver is a rather unreactive metal. This is because its filled 4d shell is not very effective in shielding the electrostatic forces of attraction from the nucleus to the outermost 5s electron, and hence silver is near the bottom of the electrochemical series (''E''0(Ag+/Ag) = +0.799 V). In group 11, silver has the lowest first ionization energy (showing the instability of the 5s orbital), but has higher second and third ionization energies than copper and gold (showing the stability of the 4d orbitals), so that the chemistry of silver is predominantly that of the +1 oxidation state, reflecting the increasingly limited range of oxidation states along the transition series as the d-orbitals fill and stabilize.Greenwood and Earnshaw, p. 1180 Unlike copper, for which the larger hydration energy of Cu2+ as compared to Cu+ is the reason why the former is the more stable in aqueous solution and solids despite lacking the stable filled d-subshell of the latter, with silver this effect is swamped by its larger second ionisation energy. Hence, Ag+ is the stable species in aqueous solution and solids, with Ag2+ being much less stable as it oxidizes water. Most silver compounds have significant covalent character due to the small size and high first ionization energy (730.8 kJ/mol) of silver. Furthermore, silver's Pauling electronegativity of 1.93 is higher than that of lead (1.87), and its electron affinity of 125.6 kJ/mol is much higher than that of hydrogen (72.8 kJ/mol) and not much less than that of oxygen (141.0 kJ/mol).Greenwood and Earnshaw, p. 1176 Due to its full d-subshell, silver in its main +1 oxidation state exhibits relatively few properties of the transition metals proper from groups 4 to 10, forming rather unstable organometallic compounds, forming linear complexes showing very low coordination numbers like 2, and forming an amphoteric oxide as well as Zintl phases like the post-transition metals. Unlike the preceding transition metals, the +1 oxidation state of silver is stable even in the absence of π-acceptor ligands. Silver does not react with air, even at red heat, and thus was considered byalchemist
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscience, protoscientific tradition that was historically practiced in Chinese alchemy, C ...
s as a noble metal, along with gold. Its reactivity is intermediate between that of copper (which forms copper(I) oxide when heated in air to red heat) and gold. Like copper, silver reacts with sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
and its compounds; in their presence, silver tarnishes in air to form the black silver sulfide
Silver sulfide is an inorganic compound with the formula . A dense black solid, it is the only sulfide of silver. It is useful as a photosensitizer in photography. It constitutes the tarnish that forms over time on silverware and other silver obje ...
(copper forms the green sulfate instead, while gold does not react). Unlike copper, silver will not react with the halogens, with the exception of fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
gas, with which it forms the difluoride
Difluorides are chemical compounds with two fluorine atoms per molecule (or per formula unit).
Metal difluorides are all ionic. Despite being highly ionic, the alkali earth metal difluorides generally have extremely high lattice stability and ...
. While silver is not attacked by non-oxidizing acids, the metal dissolves readily in hot concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
, as well as dilute or concentrated nitric acid. In the presence of air, and especially in the presence of hydrogen peroxide, silver dissolves readily in aqueous solutions of cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
.Greenwood and Earnshaw, p. 1179
The three main forms of deterioration in historical silver artifacts are tarnishing, formation of silver chloride due to long-term immersion in salt water, as well as reaction with nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
ions or oxygen. Fresh silver chloride is pale yellow, becoming purplish on exposure to light; it projects slightly from the surface of the artifact or coin. The precipitation of copper in ancient silver can be used to date artifacts, as copper is nearly always a constituent of silver alloys.
Silver metal is attacked by strong oxidizers such as potassium permanganate () and potassium dichromate (), and in the presence of potassium bromide
Potassium bromide ( K Br) is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the US. Its action is due to the bromide ion (sodium bromide is equall ...
(). These compounds are used in photography to bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
silver images, converting them to silver bromide that can either be fixed with thiosulfate or redeveloped to intensify the original image. Silver forms cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
complexes (silver cyanide
Silver cyanide is the chemical compound with the formula AgCN. It is a white solid that precipitated upon treatment of solutions containing Ag+ with cyanide, which is used in some schemes to recover silver from solution. Silver cyanide is used in ...
) that are soluble in water in the presence of an excess of cyanide ions. Silver cyanide solutions are used in electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be ...
of silver.
The common oxidation states of silver are (in order of commonness): +1 (the most stable state; for example, silver nitrate, AgNO3); +2 (highly oxidising; for example, silver(II) fluoride
Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound. Silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.
Preparation
AgF2 can be synthesized by fluori ...
, AgF2); and even very rarely +3 (extreme oxidising; for example, potassium tetrafluoroargentate(III), KAgF4). The +3 state requires very strong oxidising agents to attain, such as fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
or peroxodisulfate, and some silver(III) compounds react with atmospheric moisture and attack glass.Greenwood and Earnshaw, p. 1188 Indeed, silver(III) fluoride is usually obtained by reacting silver or silver monofluoride with the strongest known oxidizing agent, krypton difluoride.Greenwood and Earnshaw, p. 903
Compounds
Oxides and chalcogenides
Silver(I) sulfide
Silver sulfide is an inorganic compound with the formula . A dense black solid, it is the only sulfide of silver. It is useful as a photosensitizer in photography. It constitutes the tarnish that forms over time on silverware and other silver ob ...
, Ag2S, is very readily formed from its constituent elements and is the cause of the black tarnish on some old silver objects. It may also be formed from the reaction of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
with silver metal or aqueous Ag+ ions. Many non-stoichiometric selenides and tellurides are known; in particular, AgTe~3 is a low-temperature superconductor.
Halides
The only known dihalide of silver is the difluoride, AgF2, which can be obtained from the elements under heat. A strong yet thermally stable and therefore safe fluorinating agent, silver(II) fluoride is often used to synthesize hydrofluorocarbons.Greenwood and Earnshaw, pp. 1183–85 In stark contrast to this, all four silver(I) halides are known. Thefluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
, chloride, and bromide have the sodium chloride structure, but the iodide has three known stable forms at different temperatures; that at room temperature is the cubic zinc blende structure. They can all be obtained by the direct reaction of their respective elements. As the halogen group is descended, the silver halide gains more and more covalent character, solubility decreases, and the color changes from the white chloride to the yellow iodide as the energy required for ligand-metal charge transfer (X−Ag+ → XAg) decreases. The fluoride is anomalous, as the fluoride ion is so small that it has a considerable solvation energy and hence is highly water-soluble and forms di- and tetrahydrates. The other three silver halides are highly insoluble in aqueous solutions and are very commonly used in gravimetric analytical methods. All four are photosensitive (though the monofluoride is so only to ultraviolet light), especially the bromide and iodide which photodecompose to silver metal, and thus were used in traditional photography. The reaction involved is:Greenwood and Earnshaw, pp. 1185–87
:X− + ''hν'' → X + e− (excitation of the halide ion, which gives up its extra electron into the conduction band)
:Ag+ + e− → Ag (liberation of a silver ion, which gains an electron to become a silver atom)
The process is not reversible because the silver atom liberated is typically found at a crystal defect or an impurity site, so that the electron's energy is lowered enough that it is "trapped".
Other inorganic compounds
White silver nitrate, AgNO3, is a versatile precursor to many other silver compounds, especially the halides, and is much less sensitive to light. It was once called ''lunar caustic'' because silver was called ''luna'' by the ancient alchemists, who believed that silver was associated with the Moon. It is often used for gravimetric analysis, exploiting the insolubility of the heavier silver halides which it is a common precursor to. Silver nitrate is used in many ways inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, e.g. for deprotection and oxidations. Ag+ binds alkenes reversibly, and silver nitrate has been used to separate mixtures of alkenes by selective absorption. The resulting adduct can be decomposed with ammonia to release the free alkene.
Yellow silver carbonate, Ag2CO3 can be easily prepared by reacting aqueous solutions of sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
with a deficiency of silver nitrate. Its principal use is for the production of silver powder for use in microelectronics. It is reduced with formaldehyde, producing silver free of alkali metals:Andreas Brumby et al. "Silver, Silver Compounds, and Silver Alloys" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2008.
:Ag2CO3 + CH2O → 2 Ag + 2 CO2 + H2
Silver carbonate is also used as a reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
in organic synthesis such as the Koenigs-Knorr reaction. In the Fétizon oxidation, silver carbonate on celite acts as an oxidising agent to form lactones from diols
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is e ...
. It is also employed to convert alkyl bromides into alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s.
Silver fulminate, AgCNO, a powerful, touch-sensitive explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An expl ...
used in percussion caps, is made by reaction of silver metal with nitric acid in the presence of ethanol. Other dangerously explosive silver compounds are silver azide
Silver azide is the chemical compound with the formula . It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. It is a well-known explosive.
Structure and chemistry
Silver azide can be prepared by treating an aqueous solution ...
, AgN3, formed by reaction of silver nitrate with sodium azide, and silver acetylide, Ag2C2, formed when silver reacts with acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
gas in ammonia solution. In its most characteristic reaction, silver azide decomposes explosively, releasing nitrogen gas: given the photosensitivity of silver salts, this behaviour may be induced by shining a light on its crystals.
: 2 (s) → 3 (g) + 2 Ag (s)
Coordination compounds
 Silver complexes tend to be similar to those of its lighter homologue copper. Silver(III) complexes tend to be rare and very easily reduced to the more stable lower oxidation states, though they are slightly more stable than those of copper(III). For instance, the square planar periodate g(IO5OH)2sup>5− and tellurate g2sup>5− complexes may be prepared by oxidising silver(I) with alkaline peroxodisulfate. The yellow diamagnetic gF4sup>− is much less stable, fuming in moist air and reacting with glass.
Silver(II) complexes are more common. Like the valence isoelectronic copper(II) complexes, they are usually square planar and paramagnetic, which is increased by the greater field splitting for 4d electrons than for 3d electrons. Aqueous Ag2+, produced by oxidation of Ag+ by ozone, is a very strong oxidising agent, even in acidic solutions: it is stabilized in phosphoric acid due to complex formation. Peroxodisulfate oxidation is generally necessary to give the more stable complexes with heterocyclic amines, such as g(py)4sup>2+ and g(bipy)2sup>2+: these are stable provided the counterion cannot reduce the silver back to the +1 oxidation state. gF4sup>2− is also known in its violet barium salt, as are some silver(II) complexes with ''N''- or ''O''-donor ligands such as pyridine carboxylates.Greenwood and Earnshaw, p. 1189
By far the most important oxidation state for silver in complexes is +1. The Ag+ cation is diamagnetic, like its homologues Cu+ and Au+, as all three have closed-shell electron configurations with no unpaired electrons: its complexes are colourless provided the ligands are not too easily polarized such as I−. Ag+ forms salts with most anions, but it is reluctant to coordinate to oxygen and thus most of these salts are insoluble in water: the exceptions are the nitrate, perchlorate, and fluoride. The tetracoordinate tetrahedral aqueous ion g(H2O)4sup>+ is known, but the characteristic geometry for the Ag+ cation is 2-coordinate linear. For example, silver chloride dissolves readily in excess aqueous ammonia to form g(NH3)2sup>+; silver salts are dissolved in photography due to the formation of the thiosulfate complex g(S2O3)2sup>3−; and
Silver complexes tend to be similar to those of its lighter homologue copper. Silver(III) complexes tend to be rare and very easily reduced to the more stable lower oxidation states, though they are slightly more stable than those of copper(III). For instance, the square planar periodate g(IO5OH)2sup>5− and tellurate g2sup>5− complexes may be prepared by oxidising silver(I) with alkaline peroxodisulfate. The yellow diamagnetic gF4sup>− is much less stable, fuming in moist air and reacting with glass.
Silver(II) complexes are more common. Like the valence isoelectronic copper(II) complexes, they are usually square planar and paramagnetic, which is increased by the greater field splitting for 4d electrons than for 3d electrons. Aqueous Ag2+, produced by oxidation of Ag+ by ozone, is a very strong oxidising agent, even in acidic solutions: it is stabilized in phosphoric acid due to complex formation. Peroxodisulfate oxidation is generally necessary to give the more stable complexes with heterocyclic amines, such as g(py)4sup>2+ and g(bipy)2sup>2+: these are stable provided the counterion cannot reduce the silver back to the +1 oxidation state. gF4sup>2− is also known in its violet barium salt, as are some silver(II) complexes with ''N''- or ''O''-donor ligands such as pyridine carboxylates.Greenwood and Earnshaw, p. 1189
By far the most important oxidation state for silver in complexes is +1. The Ag+ cation is diamagnetic, like its homologues Cu+ and Au+, as all three have closed-shell electron configurations with no unpaired electrons: its complexes are colourless provided the ligands are not too easily polarized such as I−. Ag+ forms salts with most anions, but it is reluctant to coordinate to oxygen and thus most of these salts are insoluble in water: the exceptions are the nitrate, perchlorate, and fluoride. The tetracoordinate tetrahedral aqueous ion g(H2O)4sup>+ is known, but the characteristic geometry for the Ag+ cation is 2-coordinate linear. For example, silver chloride dissolves readily in excess aqueous ammonia to form g(NH3)2sup>+; silver salts are dissolved in photography due to the formation of the thiosulfate complex g(S2O3)2sup>3−; and cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
extraction for silver (and gold) works by the formation of the complex g(CN)2sup>−. Silver cyanide forms the linear polymer ; silver thiocyanate has a similar structure, but forms a zigzag instead because of the sp3- hybridized sulfur atom. Chelating ligand
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
s are unable to form linear complexes and thus silver(I) complexes with them tend to form polymers; a few exceptions exist, such as the near-tetrahedral diphosphine and diarsine complexes g(L–L)2sup>+.Greenwood and Earnshaw, pp. 1195–96
Organometallic
Under standard conditions, silver does not form simple carbonyls, due to the weakness of the Ag–C bond. A few are known at very low temperatures around 6–15 K, such as the green, planar paramagnetic Ag(CO)3, which dimerizes at 25–30 K, probably by forming Ag–Ag bonds. Additionally, the silver carbonyl g(CO) (OTeF5)4is known. Polymeric AgLX complexes with alkenes andalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s are known, but their bonds are thermodynamically weaker than even those of the platinum complexes (though they are formed more readily than those of the analogous gold complexes): they are also quite unsymmetrical, showing the weak ''π'' bonding in group 11. Ag–C ''σ'' bonds may also be formed by silver(I), like copper(I) and gold(I), but the simple alkyls and aryls of silver(I) are even less stable than those of copper(I) (which tend to explode under ambient conditions). For example, poor thermal stability is reflected in the relative decomposition temperatures of AgMe (−50 °C) and CuMe (−15 °C) as well as those of PhAg (74 °C) and PhCu (100 °C).Greenwood and Earnshaw, pp. 1199–200
The C–Ag bond is stabilized by perfluoroalkyl ligands, for example in AgCF(CF3)2. Alkenylsilver compounds are also more stable than their alkylsilver counterparts. Silver- NHC complexes are easily prepared, and are commonly used to prepare other NHC complexes by displacing labile ligands. For example, the reaction of the bis(NHC)silver(I) complex with bis(acetonitrile)palladium dichloride
Bis(acetonitrile)palladium dichloride is the coordination complex with the formula PdCl2(NCCH3)2. It is the adduct of two acetonitrile ligands with palladium(II) chloride. It is a yellow-brown solid that is soluble in organic solvents. The comp ...
or chlorido(dimethyl sulfide)gold(I):
:
Intermetallic
boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
and carbon) have very complex Ag–M phase diagrams and form the most commercially important alloys; and the remaining elements on the periodic table have no consistency in their Ag–M phase diagrams. By far the most important such alloys are those with copper: most silver used for coinage and jewellery is in reality a silver–copper alloy, and the eutectic mixture is used in vacuum brazing. The two metals are completely miscible as liquids but not as solids; their importance in industry comes from the fact that their properties tend to be suitable over a wide range of variation in silver and copper concentration, although most useful alloys tend to be richer in silver than the eutectic mixture (71.9% silver and 28.1% copper by weight, and 60.1% silver and 28.1% copper by atom).Ullmann, pp. 54–61
Most other binary alloys are of little use: for example, silver–gold alloys are too soft and silver– cadmium alloys too toxic. Ternary alloys have much greater importance: dental amalgams
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital, an in ...
are usually silver–tin–mercury alloys, silver–copper–gold alloys are very important in jewellery (usually on the gold-rich side) and have a vast range of hardnesses and colours, silver–copper–zinc alloys are useful as low-melting brazing alloys, and silver–cadmium– indium (involving three adjacent elements on the periodic table) is useful in nuclear reactors because of its high thermal neutron capture cross-section
Cross section may refer to:
* Cross section (geometry)
** Cross-sectional views in architecture & engineering 3D
*Cross section (geology)
* Cross section (electronics)
* Radar cross section, measure of detectability
* Cross section (physics)
**Ab ...
, good conduction of heat, mechanical stability, and resistance to corrosion in hot water.
Etymology
The word "silver" appears inOld English
Old English (, ), or Anglo-Saxon, is the earliest recorded form of the English language, spoken in England and southern and eastern Scotland in the early Middle Ages. It was brought to Great Britain by Anglo-Saxon settlement of Britain, Anglo ...
in various spellings, such as ''seolfor'' and ''siolfor''. It is cognate
In historical linguistics, cognates or lexical cognates are sets of words in different languages that have been inherited in direct descent from an etymology, etymological ancestor in a proto-language, common parent language. Because language c ...
with Old High German ''silabar''; Gothic ''silubr''; or Old Norse ''silfr'', all ultimately deriving from Proto-Germanic ''*silubra''. The Balto-Slavic words for silver are rather similar to the Germanic ones (e.g. Russian серебро 'serebró'' Polish ''srebro'', Lithuanian
Lithuanian may refer to:
* Lithuanians
* Lithuanian language
* The country of Lithuania
* Grand Duchy of Lithuania
* Culture of Lithuania
* Lithuanian cuisine
* Lithuanian Jews as often called "Lithuanians" (''Lita'im'' or ''Litvaks'') by other Jew ...
''sidãbras''), as is the Celtiberian form ''silabur''. They may have a common Indo-European origin, although their morphology rather suggest a non-Indo-European '' Wanderwort''. Some scholars have thus proposed a Paleo-Hispanic origin, pointing to the Basque form ''zilharr'' as an evidence.
The chemical symbol Ag is from the Latin word for "silver", ''argentum'' (compare Ancient Greek ἄργυρος, ''árgyros''), from the Proto-Indo-European root *''h₂erǵ-'' (formerly reconstructed as ''*arǵ-''), meaning "white" or "shining". This was the usual Proto-Indo-European word for the metal, whose reflexes are missing in Germanic and Balto-Slavic.
History
 Silver was one of the seven
Silver was one of the seven metals of antiquity
The metals of antiquity are the seven metals which humans had identified and found use for in prehistoric times in Europe and the Middle East: gold, silver, copper, tin, lead, iron, and mercury. These seven are the metals from which the moder ...
that were known to prehistoric humans and whose discovery is thus lost to history.Weeks, p. 4 In particular, the three metals of group 11, copper, silver, and gold, occur in the elemental form in nature and were probably used as the first primitive forms of money as opposed to simple bartering.Greenwood and Earnshaw, pp. 1173–74 However, unlike copper, silver did not lead to the growth of metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the sc ...
on account of its low structural strength, and was more often used ornamentally or as money. Since silver is more reactive than gold, supplies of native silver were much more limited than those of gold. For example, silver was more expensive than gold in Egypt until around the fifteenth century BC:Weeks, pp. 14–19 the Egyptians are thought to have separated gold from silver by heating the metals with salt, and then reducing the silver chloride produced to the metal.
The situation changed with the discovery of cupellation, a technique that allowed silver metal to be extracted from its ores. While slag
Slag is a by-product of smelting (pyrometallurgical) ores and used metals. Broadly, it can be classified as ferrous (by-products of processing iron and steel), ferroalloy (by-product of ferroalloy production) or non-ferrous/base metals (by-prod ...
heaps found in Asia Minor and on the islands of the Aegean Sea indicate that silver was being separated from lead as early as the 4th millennium BC
The 4th millennium BC spanned the years 4000 BC to 3001 BC. Some of the major changes in human culture during this time included the beginning of the Bronze Age and the invention of writing, which played a major role in starting recorded history. ...
, and one of the earliest silver extraction centres in Europe was Sardinia in the early Chalcolithic period, these techniques did not spread widely until later,
when it spread throughout the region and beyond. The origins of silver production in India, China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's most populous country, with a population exceeding 1.4 billion, slightly ahead of India. China spans the equivalent of five time zones and ...
, and Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
were almost certainly equally ancient, but are not well-documented due to their great age.
 When the Phoenicians first came to what is now Spain, they obtained so much silver that they could not fit it all on their ships, and as a result used silver to weight their anchors instead of lead. By the time of the Greek and Roman civilizations, silver coins were a staple of the economy: the Greeks were already extracting silver from
When the Phoenicians first came to what is now Spain, they obtained so much silver that they could not fit it all on their ships, and as a result used silver to weight their anchors instead of lead. By the time of the Greek and Roman civilizations, silver coins were a staple of the economy: the Greeks were already extracting silver from galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It cryst ...
by the 7th century BC, and the rise of Athens was partly made possible by the nearby silver mines at Laurium, from which they extracted about 30 tonnes a year from 600 to 300 BC. The stability of the Roman currency
Roman currency for most of Roman history consisted of gold, silver, bronze, orichalcum and copper coinage. From its introduction to the Republic, during the third century BC, well into Imperial times, Roman currency saw many changes in form, denomi ...
relied to a high degree on the supply of silver bullion, mostly from Spain, which Roman miners produced on a scale unparalleled before the discovery of the New World
During the Age of Discovery, a large scale European colonization of the Americas took place between about 1492 and 1800. Although the Norse had explored and colonized areas of the North Atlantic, colonizing Greenland and creating a short ter ...
. Reaching a peak production of 200 tonnes per year, an estimated silver stock of 10,000 tonnes circulated in the Roman economy in the middle of the second century AD, five to ten times larger than the combined amount of silver available to medieval Europe and the Abbasid Caliphate around AD 800. The Romans also recorded the extraction of silver in central and northern Europe in the same time period. This production came to a nearly complete halt with the fall of the Roman Empire, not to resume until the time of Charlemagne: by then, tens of thousands of tonnes of silver had already been extracted.Ullmann, pp. 16–19
Central Europe became the centre of silver production during the Middle Ages, as the Mediterranean deposits exploited by the ancient civilisations had been exhausted. Silver mines were opened in Bohemia
Bohemia ( ; cs, Čechy ; ; hsb, Čěska; szl, Czechy) is the westernmost and largest historical region of the Czech Republic. Bohemia can also refer to a wider area consisting of the historical Lands of the Bohemian Crown ruled by the Bohem ...
, Saxony, Erzgebirge, Alsace, the Lahn region, Siegerland
The Siegerland is a region of Germany covering the old district of Siegen (now part of the district of Siegen-Wittgenstein in North Rhine-Westphalia) and the upper part of the district of Altenkirchen, belonging to the Rhineland-Palatinate adjoin ...
, Silesia, Hungary, Norway, Steiermark
Styria (german: Steiermark ; Serbo-Croatian and sl, ; hu, Stájerország) is a state (''Bundesland'') in the southeast of Austria. With an area of , Styria is the second largest state of Austria, after Lower Austria. Styria is bordered to ...
, Schwaz, and the southern Black Forest. Most of these ores were quite rich in silver and could simply be separated by hand from the remaining rock and then smelted; some deposits of native silver were also encountered. Many of these mines were soon exhausted, but a few of them remained active until the Industrial Revolution, before which the world production of silver was around a meagre 50 tonnes per year. In the Americas, high temperature silver-lead cupellation technology was developed by pre-Inca civilizations as early as AD 60–120; silver deposits in India, China, Japan, and pre-Columbian America continued to be mined during this time.
With the discovery of America and the plundering of silver by the Spanish conquistadors, Central and South America became the dominant producers of silver until around the beginning of the 18th century, particularly Peru, Bolivia
, image_flag = Bandera de Bolivia (Estado).svg
, flag_alt = Horizontal tricolor (red, yellow, and green from top to bottom) with the coat of arms of Bolivia in the center
, flag_alt2 = 7 × 7 square p ...
, Chile, and Argentina: the last of these countries later took its name from that of the metal that composed so much of its mineral wealth. The silver trade gave way to a global network of exchange. As one historian put it, silver "went round the world and made the world go round." Much of this silver ended up in the hands of the Chinese. A Portuguese merchant in 1621 noted that silver "wanders throughout all the world... before flocking to China, where it remains as if at its natural center." Still, much of it went to Spain, allowing Spanish rulers to pursue military and political ambitions in both Europe and the Americas. "New World mines," concluded several historians, "supported the Spanish empire."
In the 19th century, primary production of silver moved to North America, particularly Canada, Mexico, and Nevada in the United States: some secondary production from lead and zinc ores also took place in Europe, and deposits in Siberia and the Russian Far East as well as in Australia
Australia, officially the Commonwealth of Australia, is a Sovereign state, sovereign country comprising the mainland of the Australia (continent), Australian continent, the island of Tasmania, and numerous List of islands of Australia, sma ...
were mined. Poland emerged as an important producer during the 1970s after the discovery of copper deposits that were rich in silver, before the centre of production returned to the Americas the following decade. Today, Peru and Mexico are still among the primary silver producers, but the distribution of silver production around the world is quite balanced and about one-fifth of the silver supply comes from recycling instead of new production.
Horus
Horus or Heru, Hor, Har in Ancient Egyptian, is one of the most significant ancient Egyptian deities who served many functions, most notably as god of kingship and the sky. He was worshipped from at least the late prehistoric Egypt until the P ...
as falcon god with an Egyptian crown; circa 500 BC; silver and electrum; height: 26.9 cm; Staatliche Sammlung für Ägyptische Kunst
The Staatliches Museum Ägyptischer Kunst (, ''State Museum of Egyptian Art'') is an archaeological museum in Munich. It contains the Bavarian state collection of ancient Egyptian art and displays exhibits from both the predynastic and dynastic p ...
( Munich, Germany)
Silver tetradrachm MET DP139641.jpg, Ancient Greek tetradrachm; 315–308 BC; diameter: 2.7 cm; Metropolitan Museum of Art
Silver-gilt bowl MET DP105813.jpg, Ancient Greek gilded bowl; 2nd–1st century BC; height: 7.6 cm, dimeter: 14.8 cm; Metropolitan Museum of Art
Silver plate MET DP231273.jpg, Roman plate; 1st–2nd century AD; height: 0.1 cm, diameter: 12.7 cm; Metropolitan Museum of Art
Silver bust of Serapis MET DT6658.jpg, Roman bust of Serapis; 2nd century; 15.6 x 9.5 cm; Metropolitan Museum of Art
Schaal met voorstellingen uit de geschiedenis van Diana en Actaeon door Paulus Willemsz van Vianen in 1613.jpg, Auricular basin with scenes from the story of Diana and Actaeon; 1613; length: 50 cm, height: 6 cm, width: 40 cm; Rijksmuseum
The Rijksmuseum () is the national museum of the Netherlands dedicated to Dutch arts and history and is located in Amsterdam. The museum is located at the Museum Square in the borough of Amsterdam South, close to the Van Gogh Museum, the St ...
( Amsterdam, the Netherlands)
Silver Tureen (a), lid (b) -pair with 1975.1.2560a-c- MET SLP2561a b-1.jpg, French Rococo tureen; 1749; height: 26.3 cm, width: 39 cm, depth: 24 cm; Metropolitan Museum of Art
Coffeepot MET DP103144 (cropped),.jpg, French Rococo coffeepot; 1757; height: 29.5 cm; Metropolitan Museum of Art
Ewer MET DT236853.jpg, French Neoclassical ewer; 1784–1785; height: 32.9 cm; Metropolitan Museum of Art
Elkington & Co. - Neo-Rococo Coffee Pot - 2003.243 - Cleveland Museum of Art.jpg, Neo-Rococo coffeepot; 1845; overall: 32 x 23.8 x 15.4 cm; Cleveland Museum of Art ( Cleveland, Ohio, USA)
Dessert Spoon (France), ca. 1890 (CH 18653899-2).jpg, French Art Nouveau
Art Nouveau (; ) is an international style of art, architecture, and applied art, especially the decorative arts. The style is known by different names in different languages: in German, in Italian, in Catalan, and also known as the Modern ...
dessert spoons; circa 1890; Cooper Hewitt, Smithsonian Design Museum (New York City)
Jardiniere And Liner (Germany), ca. 1905–10 (CH 18444035) (cropped).jpg, Art Nouveau jardinière; circa 1905–1910; height: 22 cm, width: 47 cm, depth: 22.5 cm; Cooper Hewitt, Smithsonian Design Museum
Handspiegel met gedreven Jugendstilornament, BK-1967-10.jpg, Hand mirror; 1906; height: 20.7 cm, weight: 88 g; Rijksmuseum
The Rijksmuseum () is the national museum of the Netherlands dedicated to Dutch arts and history and is located in Amsterdam. The museum is located at the Museum Square in the borough of Amsterdam South, close to the Van Gogh Museum, the St ...
( Amsterdam, the Netherlands)
Mystery watch.jpg, Mystery watch
A mystery watch (same applies for mystery clock) is a generic term used in horology to describe a timepiece whose working is not easily deducible, because it seems to have no movement at all, or the hands do not seem to be connected to any movem ...
; ca. 1889; diameter: 5.4 cm, depth: 1.8 cm; Musée d'Horlogerie of Le Locle, (Switzerland
). Swiss law does not designate a ''capital'' as such, but the federal parliament and government are installed in Bern, while other federal institutions, such as the federal courts, are in other cities (Bellinzona, Lausanne, Luzern, Neuchâtel ...
)
Symbolic role
Hesiod
Hesiod (; grc-gre, Ἡσίοδος ''Hēsíodos'') was an ancient Greek poet generally thought to have been active between 750 and 650 BC, around the same time as Homer. He is generally regarded by western authors as 'the first written poet i ...
's '' Works and Days'' (lines 109–201) lists different ages of man named after metals like gold, silver, bronze and iron to account for successive ages of humanity. Ovid's '' Metamorphoses'' contains another retelling of the story, containing an illustration of silver's metaphorical use of signifying the second-best in a series, better than bronze but worse than gold:
In folklore, silver was commonly thought to have mystic powers: for example, a bullet cast from silver is often supposed in such folklore the only weapon that is effective against a werewolf, witch, or other monster
A monster is a type of fictional creature found in horror, fantasy, science fiction, folklore, mythology and religion. Monsters are very often depicted as dangerous and aggressive with a strange, grotesque appearance that causes terror and fe ...
s. From this the idiom of a silver bullet developed into figuratively referring to any simple solution with very high effectiveness or almost miraculous results, as in the widely discussed software engineering paper '' No Silver Bullet''. Other powers attributed to silver include detection of poison and facilitation of passage into the mythical realm of fairies.
Silver production has also inspired figurative language. Clear references to cupellation occur throughout the Old Testament
The Old Testament (often abbreviated OT) is the first division of the Christian biblical canon, which is based primarily upon the 24 books of the Hebrew Bible or Tanakh, a collection of ancient religious Hebrew writings by the Israelites. The ...
of the Bible, such as in Jeremiah's rebuke to Judah: "The bellows are burned, the lead is consumed of the fire; the founder melteth in vain: for the wicked are not plucked away. Reprobate silver shall men call them, because the Lord hath rejected them." (Jeremiah 6:19–20) Jeremiah was also aware of sheet silver, exemplifying the malleability and ductility of the metal: "Silver spread into plates is brought from Tarshish, and gold from Uphaz, the work of the workman, and of the hands of the founder: blue and purple is their clothing: they are all the work of cunning men." (Jeremiah 10:9)
Silver also has more negative cultural meanings: the idiom thirty pieces of silver, referring to a reward for betrayal, references the bribe Judas Iscariot is said in the New Testament to have taken from Jewish leaders in Jerusalem to turn Jesus of Nazareth
Jesus, likely from he, יֵשׁוּעַ, translit=Yēšūaʿ, label=Hebrew/Aramaic ( AD 30 or 33), also referred to as Jesus Christ or Jesus of Nazareth (among other names and titles), was a first-century Jewish preacher and religious ...
over to soldiers of the high priest Caiaphas. Ethically, silver also symbolizes greed and degradation of consciousness; this is the negative aspect, the perverting of its value.
Occurrence and production
The abundance of silver in the Earth's crust is 0.08 parts per million, almost exactly the same as that ofmercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
. It mostly occurs in sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
ores, especially acanthite and argentite, Ag2S. Argentite deposits sometimes also contain native silver when they occur in reducing environments, and when in contact with salt water they are converted to chlorargyrite
Chlorargyrite is the mineral form of silver chloride (AgCl). Chlorargyrite occurs as a secondary mineral phase in the oxidation of silver mineral deposits. It crystallizes in the isometric - hexoctahedral crystal class. Typically massive to colum ...
(including horn silver), AgCl, which is prevalent in Chile and New South Wales.Greenwood and Earnshaw, pp. 1174–67 Most other silver minerals are silver pnictides or chalcogenides; they are generally lustrous semiconductors. Most true silver deposits, as opposed to argentiferous deposits of other metals, came from Tertiary period vulcanism.Ullmann, pp. 21–22
The principal sources of silver are the ores of copper, copper-nickel, lead, and lead-zinc obtained from Peru, Bolivia
, image_flag = Bandera de Bolivia (Estado).svg
, flag_alt = Horizontal tricolor (red, yellow, and green from top to bottom) with the coat of arms of Bolivia in the center
, flag_alt2 = 7 × 7 square p ...
, Mexico, China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's most populous country, with a population exceeding 1.4 billion, slightly ahead of India. China spans the equivalent of five time zones and ...
, Australia
Australia, officially the Commonwealth of Australia, is a Sovereign state, sovereign country comprising the mainland of the Australia (continent), Australian continent, the island of Tasmania, and numerous List of islands of Australia, sma ...
, Chile, Poland and Serbia. Peru, Bolivia and Mexico have been mining silver since 1546, and are still major world producers. Top silver-producing mines are Cannington (Australia), Fresnillo (Mexico), San Cristóbal (Bolivia), Antamina
The Antamina mine in the Andes Mountains of Peru is one of the largest copper/zinc mines in the world. It is an open pit mine owned by Teck Resources which had an estimated life of mine at 15 years. It also produced molybdenum. It produced 390,800 ...
(Peru), Rudna (Poland), and Penasquito (Mexico). Top near-term mine development projects through 2015 are Pascua Lama (Chile), Navidad (Argentina), Jaunicipio (Mexico), Malku Khota (Bolivia), and Hackett River (Canada). In Central Asia, Tajikistan is known to have some of the largest silver deposits in the world.
Silver is usually found in nature combined with other metals, or in minerals that contain silver compounds, generally in the form of sulfides
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds la ...
such as galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It cryst ...
(lead sulfide) or cerussite (lead carbonate). So the primary production of silver requires the smelting and then cupellation of argentiferous lead ores, a historically important process.Kassianidou, V. 2003. Early Extraction of Silver from Complex Polymetallic Ores, in Craddock, P.T. and Lang, J (eds) Mining and Metal production through the Ages. London, British Museum Press: 198–206 Lead melts at 327 °C, lead oxide at 888 °C and silver melts at 960 °C. To separate the silver, the alloy is melted again at the high temperature of 960 °C to 1000 °C in an oxidizing environment. The lead oxidises to lead monoxide
Lead(II) oxide, also called lead monoxide, is the inorganic compound with the molecular formula Pb O. PbO occurs in two polymorphs: litharge having a tetragonal crystal structure, and massicot having an orthorhombic crystal structure. Modern ap ...
, then known as litharge, which captures the oxygen from the other metals present. The liquid lead oxide is removed or absorbed by capillary action into the hearth linings.
Bayley, J., Crossley, D. and Ponting, M. (eds). 2008. "Metals and Metalworking. A research framework for archaeometallurgy". Historical Metallurgy Society 6.
: (s) + 2(s) + (g) → 2(absorbed) + Ag(l)
Today, silver metal is primarily produced instead as a secondary byproduct of electrolytic refining of copper, lead, and zinc, and by application of the Parkes process on lead bullion from ore that also contains silver. In such processes, silver follows the non-ferrous metal in question through its concentration and smelting, and is later purified out. For example, in copper production, purified copper is electrolytically deposited on the cathode, while the less reactive precious metals such as silver and gold collect under the anode as the so-called "anode slime". This is then separated and purified of base metals by treatment with hot aerated dilute sulfuric
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
acid and heating with lime or silica flux, before the silver is purified to over 99.9% purity via electrolysis in nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
solution.
Commercial-grade fine silver is at least 99.9% pure, and purities greater than 99.999% are available. In 2014, Mexico was the top producer of silver (5,000 tonnes or 18.7% of the world's total of 26,800 t), followed by China (4,060 t) and Peru (3,780 t).
In marine environments
Silver concentration is low in seawater (pmol/L). Levels vary by depth and between water bodies. Dissolved silver concentrations range from 0.3 pmol/L in coastal surface waters to 22.8 pmol/L in pelagic deep waters. Analyzing the presence and dynamics of silver in marine environments is difficult due to these particularly low concentrations and complex interactions in the environment. Although a rare trace metal, concentrations are greatly impacted by fluvial, aeolian, atmospheric, and upwelling inputs, as well as anthropogenic inputs via discharge, waste disposal, and emissions from industrial companies. Other internal processes such as decomposition of organic matter may be a source of dissolved silver in deeper waters, which feeds into some surface waters through upwelling and vertical mixing. In the Atlantic and Pacific, silver concentrations are minimal at the surface but rise in deeper waters. Silver is taken up by plankton in the photic zone, remobilized with depth, and enriched in deep waters. Silver is transported from the Atlantic to the other oceanic water masses. In North Pacific waters, silver is remobilized at a slower rate and increasingly enriched compared to deep Atlantic waters. Silver has increasing concentrations that follow the major oceanic conveyor belt that cycles water and nutrients from the North Atlantic to the South Atlantic to the North Pacific. There is not an extensive amount of data focused on how marine life is affected by silver despite the likely deleterious effects it could have on organisms throughbioaccumulation
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance at a rate faster than that at which the substance is lost or eliminated ...
, association with particulate matters, and sorption. Not until about 1984 did scientists begin to understand the chemical characteristics of silver and the potential toxicity. In fact, mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
is the only other trace metal that surpasses the toxic effects of silver; however, the full extent of silver toxicity is not expected in oceanic conditions because of its ability to transfer into nonreactive biological compounds.
In one study, the presence of excess ionic silver and silver nanoparticles caused bioaccumulation effects on zebrafish organs and altered the chemical pathways within their gills. In addition, very early experimental studies demonstrated how the toxic effects of silver fluctuate with salinity and other parameters, as well as between life stages and different species such as finfish, molluscs, and crustaceans. Another study found raised concentrations of silver in the muscles and liver of dolphins and whales, indicating pollution of this metal within recent decades. Silver is not an easy metal for an organism to eliminate and elevated concentrations can cause death.
Monetary use
 The earliest known coins were minted in the kingdom of
The earliest known coins were minted in the kingdom of Lydia
Lydia (Lydian language, Lydian: 𐤮𐤱𐤠𐤭𐤣𐤠, ''Śfarda''; Aramaic: ''Lydia''; el, Λυδία, ''Lȳdíā''; tr, Lidya) was an Iron Age Monarchy, kingdom of western Asia Minor located generally east of ancient Ionia in the mod ...
in Asia Minor around 600 BC. The coins of Lydia were made of electrum, which is a naturally occurring alloy of gold and silver, that was available within the territory of Lydia. Since that time, silver standards, in which the standard economic unit of account is a fixed weight of silver, have been widespread throughout the world until the 20th century. Notable silver coins through the centuries include the Greek drachma, the Roman denarius, the Islamic dirham
The dirham, dirhem or dirhm ( ar, درهم) is a silver unit of currency historically and currently used by several Arab and Arab influenced states. The term has also been used as a related unit of mass.
Unit of mass
The dirham was a un ...
,'' Oxford English Dictionary'', 1st editions.v. 'dirhem'
the karshapana from ancient India and
rupee
Rupee is the common name for the currencies of
India, Mauritius, Nepal, Pakistan, Seychelles, and Sri Lanka, and of former currencies of Afghanistan, Bahrain, Kuwait, Oman, the United Arab Emirates (as the Gulf rupee), British East Africa, B ...
from the time of the Mughal Empire (grouped with copper and gold coins to create a trimetallic standard), and the Spanish dollar.
The ratio between the amount of silver used for coinage and that used for other purposes has fluctuated greatly over time; for example, in wartime, more silver tends to have been used for coinage to finance the war.Ullmann, pp. 63–65
Today, silver bullion has the ISO 4217
ISO 4217 is a standard published by the International Organization for Standardization (ISO) that defines alpha codes and numeric codes for the representation of currencies and provides information about the relationships between individual cu ...
currency code XAG, one of only four precious metal
Precious metals are rare, naturally occurring metallic chemical elements of high economic value.
Chemically, the precious metals tend to be less reactive than most elements (see noble metal). They are usually ductile and have a high lustre. ...
s to have one (the others being palladium, platinum, and gold). Silver coins are produced from cast rods or ingots, rolled to the correct thickness, heat-treated, and then used to cut planchet, blanks from. These blanks are then milled and minted in a coining press; modern coining presses can produce 8000 silver coins per hour.
Price
 Silver prices are normally quoted in Troy weight, troy ounces. One troy ounce is equal to . The London silver fix is published every working day at noon London time. This price is determined by several major international banks and is used by London bullion market members for trading that day. Prices are most commonly shown as the United States dollar (USD), the Pound sterling (GBP), and the Euro (EUR).
Silver prices are normally quoted in Troy weight, troy ounces. One troy ounce is equal to . The London silver fix is published every working day at noon London time. This price is determined by several major international banks and is used by London bullion market members for trading that day. Prices are most commonly shown as the United States dollar (USD), the Pound sterling (GBP), and the Euro (EUR).
Applications
Jewellery and silverware
 The major use of silver besides coinage throughout most of history was in the manufacture of jewellery and other general-use items, and this continues to be a major use today. Examples include household silver, table silver for cutlery, for which silver is highly suited due to its antibacterial properties. Western concert flutes are usually plated with or made out of sterling silver;Ullmann, pp. 65–67 in fact, most silverware is only silver-plated rather than made out of pure silver; the silver is normally put in place by
The major use of silver besides coinage throughout most of history was in the manufacture of jewellery and other general-use items, and this continues to be a major use today. Examples include household silver, table silver for cutlery, for which silver is highly suited due to its antibacterial properties. Western concert flutes are usually plated with or made out of sterling silver;Ullmann, pp. 65–67 in fact, most silverware is only silver-plated rather than made out of pure silver; the silver is normally put in place by electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be ...
. Silver-plated glass (as opposed to metal) is used for mirrors, vacuum flasks, and Christmas tree decorations.
Because pure silver is very soft, most silver used for these purposes is alloyed with copper, with finenesses of 925/1000, 835/1000, and 800/1000 being common. One drawback is the easy tarnishing of silver in the presence of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
and its derivatives. Including precious metals such as palladium, platinum, and gold gives resistance to tarnishing but is quite costly; base metals like zinc, cadmium, silicon, and germanium do not totally prevent corrosion and tend to affect the lustre and colour of the alloy. Electrolytically refined pure silver plating is effective at increasing resistance to tarnishing. The usual solutions for restoring the lustre of tarnished silver are dipping baths that reduce the silver sulfide surface to metallic silver, and cleaning off the layer of tarnish with a paste; the latter approach also has the welcome side effect of polishing the silver concurrently.
Medicine
In medicine, silver is incorporated into wound dressings and used as an antibiotic coating in medical devices. Wound dressings containing silver sulfadiazine or Silver nanoparticles, silver nanomaterials are used to treat external infections. Silver is also used in some medical applications, such as urinary catheters (where tentative evidence indicates it reduces catheter-related urinary tract infections) and in endotracheal tube, endotracheal breathing tubes (where evidence suggests it reduces ventilator-associated pneumonia). The silver ion is Biological activity, bioactive and in sufficient concentration readily kills bacteria ''in vitro''. Silver ions interfere with enzymes in the bacteria that transport nutrients, form structures, and synthesise cell walls; these ions also bond with the bacteria's genetic material. Silver and silver nanoparticles are used as an antimicrobial in a variety of industrial, healthcare, and domestic application: for example, infusing clothing with nanosilver particles thus allows them to stay odourless for longer. Bacteria can, however, develop resistance to the antimicrobial action of silver. Silver compounds are taken up by the body likemercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
compounds, but lack the toxicity of the latter. Silver and its alloys are used in cranial surgery to replace bone, and silver–tin–mercury amalgams are used in dentistry.Ullmann, pp. 67–71 Silver diammine fluoride, the fluoride salt of a coordination complex with the formula [Ag(NH3)2]F, is a topical medicament (drug) used to treat and prevent dental caries (cavities) and relieve dentinal hypersensitivity.
Electronics
Silver is very important in electronics for conductors and electrodes on account of its high electrical conductivity even when tarnished. Bulk silver and silver foils were used to make vacuum tubes, and continue to be used today in the manufacture of semiconductor devices, circuits, and their components. For example, silver is used in high quality connectors for Radio frequency, RF, Very high frequency, VHF, and higher frequencies, particularly in tuned circuits such as RF and microwave filter#Cavity filters, cavity filters where conductors cannot be scaled by more than 6%. Printed circuit board, Printed circuits and RFID antennas are made with silver paints, Powdered silver and its alloys are used in paste preparations for conductor layers and electrodes, ceramic capacitors, and other ceramic components.Ullmann, pp. 71–78Brazing alloys
Silver-containing brazing alloys are used for brazing metallic materials, mostly cobalt, nickel, and copper-based alloys, tool steels, and precious metals. The basic components are silver and copper, with other elements selected according to the specific application desired: examples include zinc, tin, cadmium, palladium, manganese, and phosphorus. Silver provides increased workability and corrosion resistance during usage.Ullmann, pp. 78–81Chemical equipment
Silver is useful in the manufacture of chemical equipment on account of its low chemical reactivity, high thermal conductivity, and being easily workable. Silver crucibles (alloyed with 0.15% nickel to avoid recrystallisation of the metal at red heat) are used for carrying out alkaline fusion. Copper and silver are also used when doing chemistry withfluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
. Equipment made to work at high temperatures is often silver-plated. Silver and its alloys with gold are used as wire or ring seals for oxygen compressors and vacuum equipment.Ullmann, pp. 81–82
Catalysis
Silver metal is a good catalyst for oxidation reactions; in fact it is somewhat too good for most purposes, as finely divided silver tends to result in complete oxidation of organic substances to carbon dioxide and water, and hence coarser-grained silver tends to be used instead. For instance, 15% silver supported on α-Al2O3 or silicates is a catalyst for the oxidation of ethylene to ethylene oxide at 230–270 °C. Dehydrogenation of methanol to formaldehyde is conducted at 600–720 °C over silver gauze or crystals as the catalyst, as is dehydrogenation of isopropanol to acetone. In the gas phase, glycol yields glyoxal and ethanol yields acetaldehyde, while organic amines are dehydrated to nitriles.Photography
The photosensitivity of the silver halides allowed for their use in traditional photography, although digital photography, which does not use silver, is now dominant. The Photographic emulsion, photosensitive emulsion used in black-and-white photography is a suspension of silver halide crystals in gelatin, possibly mixed in with some noble metal compounds for improved photosensitivity, Photographic processing, developing, and . Color photography, Colour photography requires the addition of special dye components and sensitisers, so that the initial black-and-white silver image couples with a different dye component. The original silver images are bleached off and the silver is then recovered and recycled. Silver nitrate is the starting material in all cases.Ullmann, p. 82 The use of silver nitrate and silver halides in photography has rapidly declined with the advent of digital technology. From the peak global demand for photographic silver in 1999 (267,000,000 troy ounces or 8,304.6 tonnes) the market contracted almost 70% by 2013.Nanoparticles
Nanosilver particles, between 10 and 100 nanometres in size, are used in many applications. They are used in conductive inks for printed electronics, and have a much lower melting point than larger silver particles of micrometre size. They are also used medicinally in antibacterials and antifungals in much the same way as larger silver particles. In addition, according to thEuropean Union Observatory for Nanomaterials (EUON)
silver nanoparticles are used both in pigments, as well as cosmetics.
Miscellanea
 Pure silver metal is used as a food colouring. It has the E number, E174 designation and is approved in the European Union. Traditional Indian and Pakistani dishes sometimes include decorative silver foil known as ''vark'', and in various other cultures, silver ''dragée'' are used to decorate cakes, cookies, and other dessert items.
Photochromic lenses include silver halides, so that ultraviolet light in natural daylight liberates metallic silver, darkening the lenses. The silver halides are reformed in lower light intensities. Colourless silver chloride films are used in Particle detector, radiation detectors. Zeolite sieves incorporating Ag+ ions are used to Desalination, desalinate seawater during rescues, using silver ions to precipitate chloride as silver chloride. Silver is also used for its antibacterial properties for water sanitisation, but the application of this is limited by limits on silver consumption. Colloidal silver is similarly used to disinfect closed swimming pools; while it has the advantage of not giving off a smell like hypochlorite treatments do, colloidal silver is not effective enough for more contaminated open swimming pools. Small silver iodide crystals are used in cloud seeding to cause rain.Ullmann, pp. 83–84
The Texas Legislature designated silver the official precious metal of Texas in 2007.
Pure silver metal is used as a food colouring. It has the E number, E174 designation and is approved in the European Union. Traditional Indian and Pakistani dishes sometimes include decorative silver foil known as ''vark'', and in various other cultures, silver ''dragée'' are used to decorate cakes, cookies, and other dessert items.
Photochromic lenses include silver halides, so that ultraviolet light in natural daylight liberates metallic silver, darkening the lenses. The silver halides are reformed in lower light intensities. Colourless silver chloride films are used in Particle detector, radiation detectors. Zeolite sieves incorporating Ag+ ions are used to Desalination, desalinate seawater during rescues, using silver ions to precipitate chloride as silver chloride. Silver is also used for its antibacterial properties for water sanitisation, but the application of this is limited by limits on silver consumption. Colloidal silver is similarly used to disinfect closed swimming pools; while it has the advantage of not giving off a smell like hypochlorite treatments do, colloidal silver is not effective enough for more contaminated open swimming pools. Small silver iodide crystals are used in cloud seeding to cause rain.Ullmann, pp. 83–84
The Texas Legislature designated silver the official precious metal of Texas in 2007.
Precautions
Silver compounds have low toxicity compared to those of most other heavy metals, as they are poorly absorbed by the human body when ingested, and that which does get absorbed is rapidly converted to insoluble silver compounds or complexed by metallothionein. However, silver fluoride and silver nitrate are caustic and can cause tissue damage, resulting in gastroenteritis, diarrhoea, falling blood pressure, cramps, paralysis, and respiratory arrest. Animals repeatedly dosed with silver salts have been observed to experience anaemia, slowed growth, necrosis of the liver, and fatty degeneration of the liver and kidneys; rats implanted with silver foil or injected with colloidal silver have been observed to develop localised tumours. Route of administration, Parenterally admistered colloidal silver causes acute silver poisoning.Ullmann, pp. 88–91 Some waterborne species are particularly sensitive to silver salts and those of the other precious metals; in most situations, however, silver does not pose serious environmental hazards. In large doses, silver and compounds containing it can be absorbed into the circulatory system and become deposited in various body tissues, leading to argyria, which results in a blue-grayish pigmentation of the skin, eyes, and mucous membranes. Argyria is rare, and so far as is known, does not otherwise harm a person's health, though it is disfiguring and usually permanent. Mild forms of argyria are sometimes mistaken for cyanosis, a blue tint on skin, caused by lack of oxygen. Metallic silver, like copper, is an antibacterial agent, which was known to the ancients and first scientifically investigated and named the oligodynamic effect by Carl Nägeli. Silver ions damage the metabolism of bacteria even at such low concentrations as 0.01–0.1 milligrams per litre; metallic silver has a similar effect due to the formation of silver oxide. This effect is lost in the presence ofsulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
due to the extreme insolubility of silver sulfide.
Some silver compounds are very explosive, such as the nitrogen compounds silver azide, silver amide, and silver fulminate, as well as silver acetylide, silver oxalate, and silver(II) oxide. They can explode on heating, force, drying, illumination, or sometimes spontaneously. To avoid the formation of such compounds, ammonia and acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
should be kept away from silver equipment. Salts of silver with strongly oxidising acids such as silver chlorate and silver nitrate can explode on contact with materials that can be readily oxidised, such as organic compounds, sulfur and soot.
See also
* Silver coin * Silver medal * Free silver * List of countries by silver production * :Silver compounds, List of silver compounds * Silver as an investment * Silverpoint drawingReferences
Sources used above
* * *Further reading
* William L. Silber, ''The Story of Silver: How the White Metal Shaped America and the Modern World.'' Princeton, NJ: Princeton University Press, 2019.External links
Silver
at ''The Periodic Table of Videos'' (University of Nottingham)
Society of American Silversmiths
The Silver Institute
A silver industry website
Samples of silver
Transport, Fate and Effects of Silver in the Environment
Bloomberg – Markets Precious and Industrial Metals – Silver
{{good article Silver, Chemical elements Transition metals Noble metals Precious metals Cubic minerals Minerals in space group 225 Electrical conductors Native element minerals E-number additives Chemical elements with face-centered cubic structure