Self-assembly Of Iron Oxide Nanocrystals2 on:
[Wikipedia]
[Google]
[Amazon]
 Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the constitutive components are molecules, the process is termed
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the constitutive components are molecules, the process is termed  Self-assembly can be classified as either static or dynamic. In ''static'' self-assembly, the ordered state forms as a system approaches equilibrium, reducing its free energy. However, in ''dynamic'' self-assembly, patterns of pre-existing components organized by specific local interactions are not commonly described as "self-assembled" by scientists in the associated disciplines. These structures are better described as "
Self-assembly can be classified as either static or dynamic. In ''static'' self-assembly, the ordered state forms as a system approaches equilibrium, reducing its free energy. However, in ''dynamic'' self-assembly, patterns of pre-existing components organized by specific local interactions are not commonly described as "self-assembled" by scientists in the associated disciplines. These structures are better described as "
 Self-assembly in the classic sense can be defined as ''the spontaneous and reversible organization of molecular units into ordered structures by non-covalent interactions''. The first property of a self-assembled system that this definition suggests is the spontaneity of the self-assembly process: the interactions responsible for the formation of the self-assembled system act on a strictly local level—in other words, ''the nanostructure builds itself''.
Although self-assembly typically occurs between weakly-interacting species, this organization may be transferred into strongly-bound
Self-assembly in the classic sense can be defined as ''the spontaneous and reversible organization of molecular units into ordered structures by non-covalent interactions''. The first property of a self-assembled system that this definition suggests is the spontaneity of the self-assembly process: the interactions responsible for the formation of the self-assembled system act on a strictly local level—in other words, ''the nanostructure builds itself''.
Although self-assembly typically occurs between weakly-interacting species, this organization may be transferred into strongly-bound
Freeview Video 'Self-Assembly: Nature's Way To Do It
', A Royal Institution Lecture by the Vega Science Trust. * Pape
Molecular Self-Assembly
* Wiki:
C2 Self Assembly from a computer programming perspective
'' * Pelesko, J.A., (2007
''Self Assembly: The Science of Things That Put Themselves Together,''
Chapman & Hall/CRC Press. * A brief page on self-assembly at the University of Delawar
Nanotechnology Cell biology Systems theory Self-organization

 Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the constitutive components are molecules, the process is termed
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the constitutive components are molecules, the process is termed molecular self-assembly
In chemistry and materials science, molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly: intramolecular and intermole ...
.
 Self-assembly can be classified as either static or dynamic. In ''static'' self-assembly, the ordered state forms as a system approaches equilibrium, reducing its free energy. However, in ''dynamic'' self-assembly, patterns of pre-existing components organized by specific local interactions are not commonly described as "self-assembled" by scientists in the associated disciplines. These structures are better described as "
Self-assembly can be classified as either static or dynamic. In ''static'' self-assembly, the ordered state forms as a system approaches equilibrium, reducing its free energy. However, in ''dynamic'' self-assembly, patterns of pre-existing components organized by specific local interactions are not commonly described as "self-assembled" by scientists in the associated disciplines. These structures are better described as "self-organized
Self-organization, also called spontaneous order in the social sciences, is a process where some form of overall order arises from local interactions between parts of an initially disordered system. The process can be spontaneous when suff ...
", although these terms are often used interchangeably.
Self-assembly in chemistry and materials science
 Self-assembly in the classic sense can be defined as ''the spontaneous and reversible organization of molecular units into ordered structures by non-covalent interactions''. The first property of a self-assembled system that this definition suggests is the spontaneity of the self-assembly process: the interactions responsible for the formation of the self-assembled system act on a strictly local level—in other words, ''the nanostructure builds itself''.
Although self-assembly typically occurs between weakly-interacting species, this organization may be transferred into strongly-bound
Self-assembly in the classic sense can be defined as ''the spontaneous and reversible organization of molecular units into ordered structures by non-covalent interactions''. The first property of a self-assembled system that this definition suggests is the spontaneity of the self-assembly process: the interactions responsible for the formation of the self-assembled system act on a strictly local level—in other words, ''the nanostructure builds itself''.
Although self-assembly typically occurs between weakly-interacting species, this organization may be transferred into strongly-bound covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
systems. An example for this may be observed in the self-assembly of polyoxometalates. Evidence suggests that such molecules assemble via a dense-phase type mechanism
Mechanism may refer to:
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that a ...
whereby small oxometalate ions first assemble non-covalently in solution, followed by a condensation reaction that covalently binds the assembled units. This process can be aided by the introduction of templating agents to control the formed species. In such a way, highly organized covalent molecules may be formed in a specific manner.
Self-assembled nano-structure is an object that appears as a result of ordering and aggregation of individual nano-scale objects guided by some physical
Physical may refer to:
*Physical examination
In a physical examination, medical examination, or clinical examination, a medical practitioner examines a patient for any possible medical signs or symptoms of a medical condition. It generally co ...
principle.
A particularly counter-intuitive example of a physical principle that can drive self-assembly is entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
maximization. Though entropy is conventionally associated with disorder, under suitable conditions entropy can drive nano-scale objects to self-assemble into target structures in a controllable way.
Another important class of self-assembly is field-directed assembly. An example of this is the phenomenon of electrostatic trapping. In this case an electric field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field fo ...
is applied between two metallic nano-electrodes. The particles present in the environment are polarized by the applied electric field. Because of dipole interaction with the electric field gradient the particles are attracted to the gap between the electrodes. Generalizations of this type approach involving different types of fields, e.g., using magnetic fields, using capillary interactions for particles trapped at interfaces, elastic interactions for particles suspended in liquid crystals have also been reported.
Regardless of the mechanism driving self-assembly, people take self-assembly approaches to materials synthesis to avoid the problem of having to construct materials one building block at a time. Avoiding one-at-a-time approaches is important because the amount of time required to place building blocks into a target structure is prohibitively difficult for structures that have macroscopic size.
Once materials of macroscopic size can be self-assembled, those materials can find use in many applications. For example, nano-structures such as nano-vacuum gaps are used for storing energy and nuclear energy conversion. Self-assembled tunable materials are promising candidates for large surface area electrodes in batteries
Battery most often refers to:
* Electric battery, a device that provides electrical power
* Battery (crime), a crime involving unlawful physical contact
Battery may also refer to:
Energy source
*Automotive battery, a device to provide power t ...
and organic photovoltaic cells, as well as for microfluidic sensors and filters.
Distinctive features
At this point, one may argue that any chemical reaction driving atoms and molecules to assemble into larger structures, such asprecipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. ...
, could fall into the category of self-assembly. However, there are at least three distinctive features that make self-assembly a distinct concept.
Order
First, the self-assembled structure must have a higherorder
Order, ORDER or Orders may refer to:
* Categorization, the process in which ideas and objects are recognized, differentiated, and understood
* Heterarchy, a system of organization wherein the elements have the potential to be ranked a number of d ...
than the isolated components, be it a shape or a particular task that the self-assembled entity may perform. This is generally not true in chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s, where an ordered state may proceed towards a disordered state depending on thermodynamic parameters.
Interactions
The second important aspect of self-assembly is the predominant role of weak interactions (e.g. Van der Waals,capillary
A capillary is a small blood vessel from 5 to 10 micrometres (μm) in diameter. Capillaries are composed of only the tunica intima, consisting of a thin wall of simple squamous endothelial cells. They are the smallest blood vessels in the body: ...
, , hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s, or entropic forces) compared to more "traditional" covalent, ionic, or metallic bonds. These weak interactions are important in materials synthesis for two reasons.
First, weak interactions take a prominent place in materials, especially in biological systems. For instance, they determine the physical properties of liquids, the solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
of solids, and the organization of molecules in biological membranes.
Second, in addition to the strength of the interactions, interactions with varying degrees of specificity can control self-assembly. Self-assembly that is mediated by DNA pairing interactions constitutes the interactions of the highest specificity that have been used to drive self-assembly. At the other extreme, the least specific interactions are possibly those provided by
emergent forces that arise from entropy maximization.
Building blocks
The third distinctive feature of self-assembly is that the building blocks are not only atoms and molecules, but span a wide range of nano- and mesoscopic structures, with different chemical compositions, functionalities, and shapes. Research into possible three-dimensional shapes of self-assembling micrites examines Platonic solids (regular polyhedral). The term ‘micrite’ was created byDARPA
The Defense Advanced Research Projects Agency (DARPA) is a research and development agency of the United States Department of Defense responsible for the development of emerging technologies for use by the military.
Originally known as the Adv ...
to refer to sub-millimeter sized microrobots, whose self-organizing abilities may be compared with those of slime mold. Recent examples of novel building blocks include polyhedra and patchy particles
Patchy particles are micron- or nanoscale colloidal particles that are anisotropically patterned, either by modification of the particle surface chemistry ("enthalpic patches"), through particle shape ("entropic patches"), or both. The particles ha ...
. Examples also included microparticles with complex geometries, such as hemispherical, dimer, discs, rods, molecules, as well as multimers. These nanoscale building blocks can in turn be synthesized through conventional chemical routes or by other self-assembly strategies such as directional entropic forces. More recently, inverse design approaches have appeared where it is possible to fix a target self-assembled behavior, and determine an appropriate building block that will realize that behavior.
Thermodynamics and kinetics
Self-assembly in microscopic systems usually starts from diffusion, followed by the nucleation of seeds, subsequent growth of the seeds, and ends at Ostwald ripening. The thermodynamic driving free energy can be eitherenthalpic
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
or entropic or both. In either the enthalpic or entropic case, self-assembly proceeds through the formation and breaking of bonds, possibly with non-traditional forms of mediation.
The kinetics of the self-assembly process is usually related to diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
, for which the absorption/adsorption rate often follows a Langmuir adsorption model
The Langmuir adsorption model explains adsorption by assuming an adsorbate behaves as an ideal gas at isothermal conditions. According to the model, adsorption and desorption are reversible processes. This model even explains the effect of pressu ...
which in the diffusion controlled concentration (relatively diluted solution) can be estimated by the Fick's laws of diffusion
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equ ...
. The desorption rate is determined by the bond strength of the surface molecules/atoms with a thermal activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
barrier. The growth rate is the competition between these two processes.
Examples
Important examples of self-assembly in materials science include the formation of molecularcrystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s, colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...
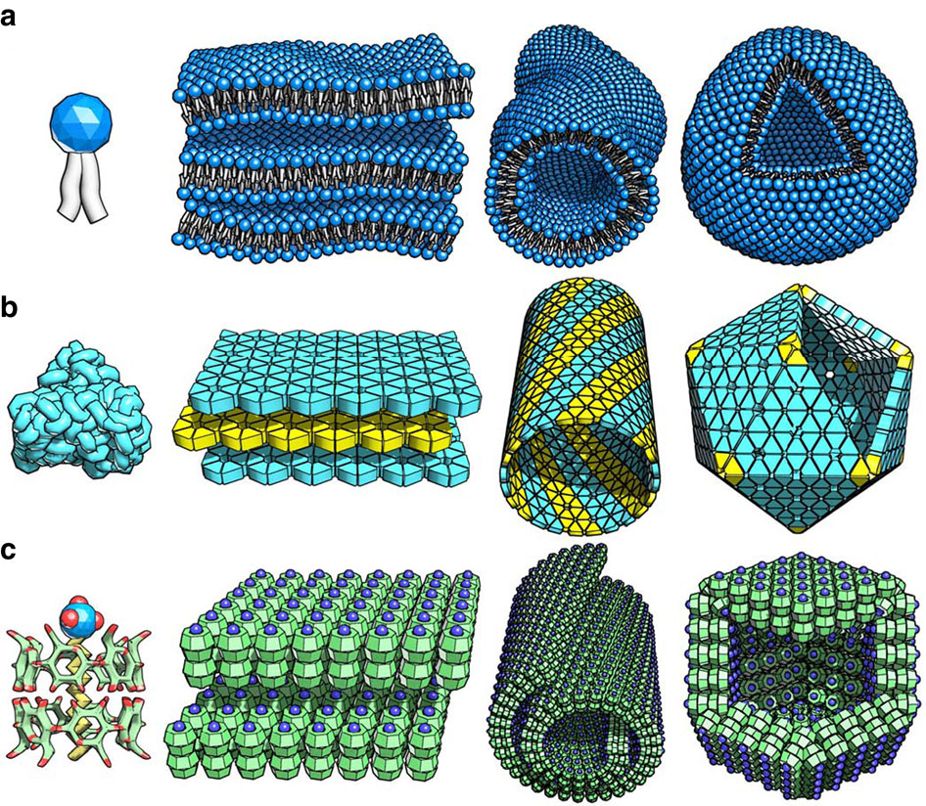
s, lipid bilayers, phase-separated polymers, and self-assembled monolayers. The folding of polypeptide chains into proteins and the folding of nucleic acids into their functional forms are examples of self-assembled biological structures. Recently, the three-dimensional macroporous structure was prepared via self-assembly of diphenylalanine derivative under cryoconditions, the obtained material can find the application in the field of regenerative medicine or drug delivery system. P. Chen et al. demonstrated a microscale self-assembly method using the air-liquid interface established by Faraday wave as a template. This self-assembly method can be used for generation of diverse sets of symmetrical and periodic patterns from microscale materials such as hydrogels, cells, and cell spheroids. Yasuga et al. demonstrated how fluid interfacial energy drives the emergence of three-dimensional periodic structures in micropillar scaffolds. Myllymäki et al. demonstrated the formation of micelles, that undergo a change in morphology to fibers and eventually to spheres, all controlled by solvent change.
Properties
Self-assembly extends the scope of chemistry aiming at synthesizing products with order and functionality properties, extending chemical bonds to weak interactions and encompassing the self-assembly of nanoscale building blocks at all length scales. In covalent synthesis and polymerization, the scientist links atoms together in any desired conformation, which does not necessarily have to be the energetically most favoured position; self-assembling molecules, on the other hand, adopt a structure at the thermodynamic minimum, finding the best combination of interactions between subunits but not forming covalent bonds between them. In self-assembling structures, the scientist must predict this minimum, not merely place the atoms in the location desired. Another characteristic common to nearly all self-assembled systems is theirthermodynamic stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system.
Thermodynamic stability occurs when a system is in its lowest energy state, or in chemical equilibrium with its environment. This may be a dynamic equilibriu ...
. For self-assembly to take place without intervention of external forces, the process must lead to a lower Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pr ...
, thus self-assembled structures are thermodynamically more stable than the single, unassembled components. A direct consequence is the general tendency of self-assembled structures to be relatively free of defects. An example is the formation of two-dimensional superlattices composed of an orderly arrangement of micrometre-sized polymethylmethacrylate (PMMA) spheres, starting from a solution containing the microspheres, in which the solvent is allowed to evaporate slowly in suitable conditions. In this case, the driving force is capillary interaction, which originates from the deformation of the surface of a liquid caused by the presence of floating or submerged particles.
These two properties—weak interactions and thermodynamic stability—can be recalled to rationalise another property often found in self-assembled systems: the ''sensitivity to perturbations'' exerted by the external environment. These are small fluctuations that alter thermodynamic variables that might lead to marked changes in the structure and even compromise it, either during or after self-assembly. The weak nature of interactions is responsible for the flexibility of the architecture and allows for rearrangements of the structure in the direction determined by thermodynamics. If fluctuations bring the thermodynamic variables back to the starting condition, the structure is likely to go back to its initial configuration. This leads us to identify one more property of self-assembly, which is generally not observed in materials synthesized by other techniques: ''reversibility''.
Self-assembly is a process which is easily influenced by external parameters. This feature can make synthesis rather complex because of the need to control many free parameters. Yet self-assembly has the advantage that a large variety of shapes and functions on many length scales can be obtained.
The fundamental condition needed for nanoscale building blocks to self-assemble into an ordered structure is the simultaneous presence of long-range repulsive and short-range attractive forces.
By choosing precursors
Precursor or Precursors may refer to:
* Precursor (religion), a forerunner, predecessor
** The Precursor, John the Baptist
Science and technology
* Precursor (bird), a hypothesized genus of fossil birds that was composed of fossilized parts of un ...
with suitable physicochemical properties, it is possible to exert a fine control on the formation processes that produce complex structures. Clearly, the most important tool when it comes to designing a synthesis strategy for a material, is the knowledge of the chemistry of the building units. For example, it was demonstrated that it was possible to use diblock copolymers with different block reactivities in order to selectively embed maghemite nanoparticles and generate periodic materials with potential use as waveguides.
In 2008 it was proposed that every self-assembly process presents a co-assembly, which makes the former term a misnomer. This thesis is built on the concept of mutual ordering of the self-assembling system and its environment.
Self-assembly at the macroscopic scale
The most common examples of self-assembly at the macroscopic scale can be seen at interfaces between gases and liquids, where molecules can be confined at the nanoscale in the vertical direction and spread over long distances laterally. Examples of self-assembly at gas-liquid interfaces include breath-figures, self-assembled monolayers, droplet clusters, andLangmuir–Blodgett film
A Langmuir–Blodgett (LB) film is a nanostructured system formed when Langmuir films—or Langmuir monolayers (LM)—are transferred from the liquid-gas interface to solid supports during the vertical passage of the support through the monolayers ...
s, while crystallization of fullerene whiskers is an example of macroscopic self-assembly in between two liquids. Another remarkable example of macroscopic self-assembly is the formation of thin quasicrystals at an air-liquid interface, which can be built up not only by inorganic, but also by organic molecular units. Furthermore, it was reported that Fmoc
The fluorenylmethoxycarbonyl protecting group (Fmoc) is a base-labile protecting group used in organic synthesis.
Protection & Formation
Fmoc carbamate is frequently used as a protecting group for amines, where the Fmoc group can be introduce ...
protected L-DOPA amino acid (Fmoc-DOPA) can present a minimal supramolecular polymer model, displaying a spontaneous structural transition from meta-stable spheres to fibrillar assemblies to gel-like material and finally to single crystals.
Self-assembly processes can also be observed in systems of macroscopic building blocks. These building blocks can be externally propelled or self-propelled. Since the 1950s, scientists have built self-assembly systems exhibiting centimeter-sized components ranging from passive mechanical parts to mobile robots. For systems at this scale, the component design can be precisely controlled. For some systems, the components' interaction preferences are programmable. The self-assembly processes can be easily monitored and analyzed by the components themselves or by external observers.
In April 2014, a 3D printed
3D printing or additive manufacturing is the construction of a three-dimensional object from a CAD model or a digital 3D model. It can be done in a variety of processes in which material is deposited, joined or solidified under computer co ...
plastic was combined with a "smart material" that self-assembles in water, resulting in "4D printing
4-dimensional printing (4D printing; also known as 4D bioprinting, active origami, or shape-morphing systems) uses the same techniques of 3D printing through computer-programmed deposition of material in successive layers to create a three-dimensio ...
".
Consistent concepts of self-organization and self-assembly
People regularly use the terms "self-organization
Self-organization, also called spontaneous order in the social sciences, is a process where some form of overall order arises from local interactions between parts of an initially disordered system. The process can be spontaneous when suffi ...
" and "self-assembly" interchangeably. As complex system
A complex system is a system composed of many components which may interact with each other. Examples of complex systems are Earth's global climate, organisms, the human brain, infrastructure such as power grid, transportation or communication ...
science becomes more popular though, there is a higher need to clearly distinguish the differences between the two mechanisms to understand their significance in physical and biological systems. Both processes explain how collective order develops from "dynamic small-scale interactions". Self-organization is a non-equilibrium process where self-assembly is a spontaneous process that leads toward equilibrium. Self-assembly requires components to remain essentially unchanged throughout the process. Besides the thermodynamic difference between the two, there is also a difference in formation. The first difference is what "encodes the global order of the whole" in self-assembly whereas in self-organization this initial encoding is not necessary. Another slight contrast refers to the minimum number of units needed to make an order. Self-organization appears to have a minimum number of units whereas self-assembly does not. The concepts may have particular application in connection with natural selection
Natural selection is the differential survival and reproduction of individuals due to differences in phenotype. It is a key mechanism of evolution, the change in the heritable traits characteristic of a population over generations. Charle ...
.
Eventually, these patterns may form one theory of pattern formation
The science of pattern formation deals with the visible, ( statistically) orderly outcomes of self-organization and the common principles behind similar patterns in nature.
In developmental biology, pattern formation refers to the generation of ...
in nature.
See also
* Crystal engineering * Autopoiesis *Langmuir–Blodgett film
A Langmuir–Blodgett (LB) film is a nanostructured system formed when Langmuir films—or Langmuir monolayers (LM)—are transferred from the liquid-gas interface to solid supports during the vertical passage of the support through the monolayers ...
* Nanotechnology
Nanotechnology, also shortened to nanotech, is the use of matter on an atomic, molecular, and supramolecular scale for industrial purposes. The earliest, widespread description of nanotechnology referred to the particular technological goal o ...
* Pick-and-place machine
Surface-mount technology (SMT) component placement systems, commonly called pick-and-place machines or P&Ps, are robotic machines which are used to place surface-mount devices (SMDs) onto a printed circuit board (PCB). They are used for high s ...
* Self-assembly of nanoparticles
Nanoparticles are classified as having at least one of three dimensions be in the range of 1-100 nm. The small size of nanoparticles allows them to have unique characteristics which may not be possible on the macro-scale. Self-assembly is t ...
*
References
Further reading
* * * * {{refendExternal links
* Kuniaki Nagayama,Freeview Video 'Self-Assembly: Nature's Way To Do It
', A Royal Institution Lecture by the Vega Science Trust. * Pape
Molecular Self-Assembly
* Wiki:
C2 Self Assembly from a computer programming perspective
'' * Pelesko, J.A., (2007
''Self Assembly: The Science of Things That Put Themselves Together,''
Chapman & Hall/CRC Press. * A brief page on self-assembly at the University of Delawar
Nanotechnology Cell biology Systems theory Self-organization