|
Hydrogels
A gel is a semi-solid that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state, although the liquid phase may still diffuse through this system. A gel has been defined phenomenologically as a soft, solid or solid-like material consisting of two or more components, one of which is a liquid, present in substantial quantity. By weight, gels are mostly liquid, yet they behave like solids because of a three-dimensional cross-linked network within the liquid. It is the crosslinking within the fluid that gives a gel its structure (hardness) and contributes to the adhesive stick (tack). In this way, gels are a dispersion of molecules of a liquid within a solid medium. The word ''gel'' was coined by 19th-century Scottish chemist Thomas Graham by clipping from ''gelatine''. The process of forming a gel is called gelation. IUPAC definition } Com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogel
A hydrogel is a crosslinked hydrophilic polymer that does not dissolve in water. They are highly absorbent yet maintain well defined structures. These properties underpin several applications, especially in the biomedical area. Many hydrogels are synthetic, but some are derived from nature. The term 'hydrogel' was coined in 1894. Chemistry Classification The crosslinks which bond the polymers of a hydrogel fall under two general categories: physical and chemical. Chemical hydrogels have covalent cross-linking bonds, whereas physical hydrogels have non-covalent bonds. Chemical hydrogels result in strong irreversible gels due to the covalent bonding, and they may also possess harmful properties which makes them unfavourable for medical applications. Physical hydrogels on the other hand have high biocompatibility, aren’t toxic, and are also easily reversible, by simply changing an external stimulus such as pH or temperature; thus they are favourable for use in medical applicat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gelation
In polymer chemistry, gelation (gel transition) is the formation of a gel from a system with polymers. Branched polymers can form links between the chains, which lead to progressively larger polymers. As the linking continues, larger branched polymers are obtained and at a certain extent of the reaction links between the polymer result in the formation of a single macroscopic molecule. At that point in the reaction, which is defined as gel point, the system loses fluidity and viscosity becomes very large. The onset of gelation, or gel point, is accompanied by a sudden increase in viscosity. This "infinite" sized polymer is called the gel or network, which does not dissolve in the solvent, but can swell in it. Background Gelation is promoted by gelling agents. Gelation can occur either by physical linking or by chemical crosslinking. While the physical gels involve physical bonds, chemical gelation involves covalent bonds. The first quantitative theories of chemical gelation w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerogel
Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Aerogels can be made from a variety of chemical compounds. Silica aerogels feel like fragile expanded polystyrene to the touch, while some polymer-based aerogels feel like rigid foams. The first documented example of an aerogel was created by Samuel Stephens Kistler in 1931, as a result of a bet with Charles Learned over who could replace the liquid in "jellies" with gas without causing shrinkage. Aerogels are produced by extracting the liquid component of a gel through supercritical drying or freeze-drying. This allows the liquid to be slowly dried off without causing the solid matrix in the gel to collapse from capillary action, as would happen with conventional evaporation. The first aer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of the molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silica (silicon dioxide, or quartz), the primary constituent of sand. Soda–lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term ''glass'', in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and eyeglasses, are so commonly made of silicate-based glass that they are simply called by the name of the material. Despite bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallinity
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a big influence on hardness, density, Transparency and translucency, transparency and diffusion. In an ideal gas, the relative positions of the atoms or molecules are completely random. Amorphous materials, such as liquids and glasses, represent an intermediate case, having order over short distances (a few atomic or molecular spacings) but not over longer distances. Many materials, such as glass-ceramics and some polymers, can be prepared in such a way as to produce a mixture of crystalline and Amorphous solid, amorphous regions. In such cases, crystallinity is usually specified as a percentage of the volume of the material that is crystalline. Even within materials that are completely crystalline, however, the degree of structural perfection can vary. For instance, most metallic alloys are crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sensors
A sensor is a device that produces an output signal for the purpose of sensing a physical phenomenon. In the broadest definition, a sensor is a device, module, machine, or subsystem that detects events or changes in its environment and sends the information to other electronics, frequently a computer processor. Sensors are always used with other electronics. Sensors are used in everyday objects such as touch-sensitive elevator buttons (tactile sensor) and lamps which dim or brighten by touching the base, and in innumerable applications of which most people are never aware. With advances in micromachinery and easy-to-use microcontroller platforms, the uses of sensors have expanded beyond the traditional fields of temperature, pressure and flow measurement, for example into MARG sensors. Analog sensors such as potentiometers and force-sensing resistors are still widely used. Their applications include manufacturing and machinery, airplanes and aerospace, cars, medicine, robot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actuators
An actuator is a component of a machine that is responsible for moving and controlling a mechanism or system, for example by opening a valve. In simple terms, it is a "mover". An actuator requires a control device (controlled by control signal) and a source of energy. The control signal is relatively low energy and may be electric voltage or current, pneumatic, or hydraulic fluid pressure, or even human power. Its main energy source may be an electric current, hydraulic pressure, or pneumatic pressure. The Control device is usually a valve. When it receives a control signal, an actuator responds by converting the source's energy into mechanical motion. In the ''electric'', ''hydraulic'', and ''pneumatic'' sense, it is a form of automation or automatic control. History The history of the pneumatic actuation system and the hydraulic actuation system dates to around the time of World War II (1938). It was first created by Xhiter Anckeleman who used his knowledge of engines and brake ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smart Materials
Smart materials, also called intelligent or responsive materials, are designed materials that have one or more properties that can be significantly changed in a controlled fashion by external stimuli, such as stress, moisture, electric or magnetic fields, light, temperature, pH, or chemical compounds. Smart materials are the basis of many applications, including sensors and actuators, or artificial muscles, particularly as electroactive polymers (EAPs). Terms used to describe smart materials include shape memory material (SMM) and shape memory technology (SMT). Types There are a number of types of smart material, of which are already common. Some examples are as following: * Piezoelectric materials are materials that produce a voltage when stress is applied. Since this effect also applies in a reverse manner, a voltage across the sample will produce stress within sample. Suitably designed structures made from these materials can, therefore, be made that bend, expand or contract ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption (chemistry)
In chemistry, absorption is a physical or chemical phenomenon or a process in which atoms, molecules or ions enter some bulk phase – liquid or solid material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume, not by the surface (as in the case for adsorption). A more common definition is that "Absorption is a chemical or physical phenomenon in which the molecules, atoms and ions of the substance getting absorbed enters into the bulk phase (gas, liquid or solid) of the material in which it is taken up." A more general term is ''sorption'', which covers absorption, adsorption, and ion exchange. Absorption is a condition in which something takes in another substance. In many processes important in technology, the chemical absorption is used in place of the physical process, e.g., absorption of carbon dioxide by sodium hydroxide – such acid-base processes do not follow the Nernst partition law (see: solubility). For ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
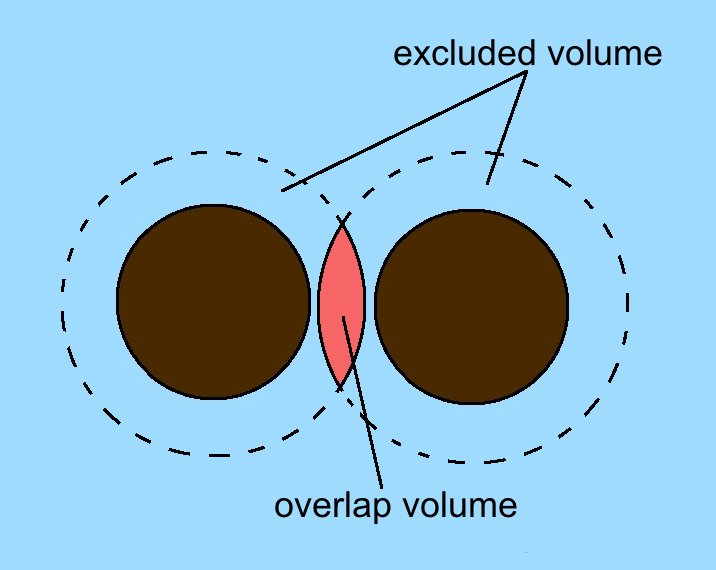
Depletion Force
A depletion force is an effective attractive force that arises between large colloidal particles that are suspended in a dilute solution of ''depletants'', which are smaller solutes that are preferentially excluded from the vicinity of the large particles. One of the earliest reports of depletion forces that lead to particle coagulation is that of Bondy, who observed the separation or "creaming" of rubber latex upon addition of polymer depletant molecules (sodium alginate) to solution. More generally, depletants can include polymers, micelles, osmolytes, ink, mud, or paint dispersed in a continuous phase. Depletion forces are often regarded as entropic forces, as was first explained by the established Asakura–Oosawa model. In this theory the depletion force arises from an increase in osmotic pressure of the surrounding solution when colloidal particles get close enough such that the excluded cosolutes (depletants) cannot fit in between them. Because the particles were consider ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |