Selective leaching on:
[Wikipedia]
[Google]
[Amazon]
In
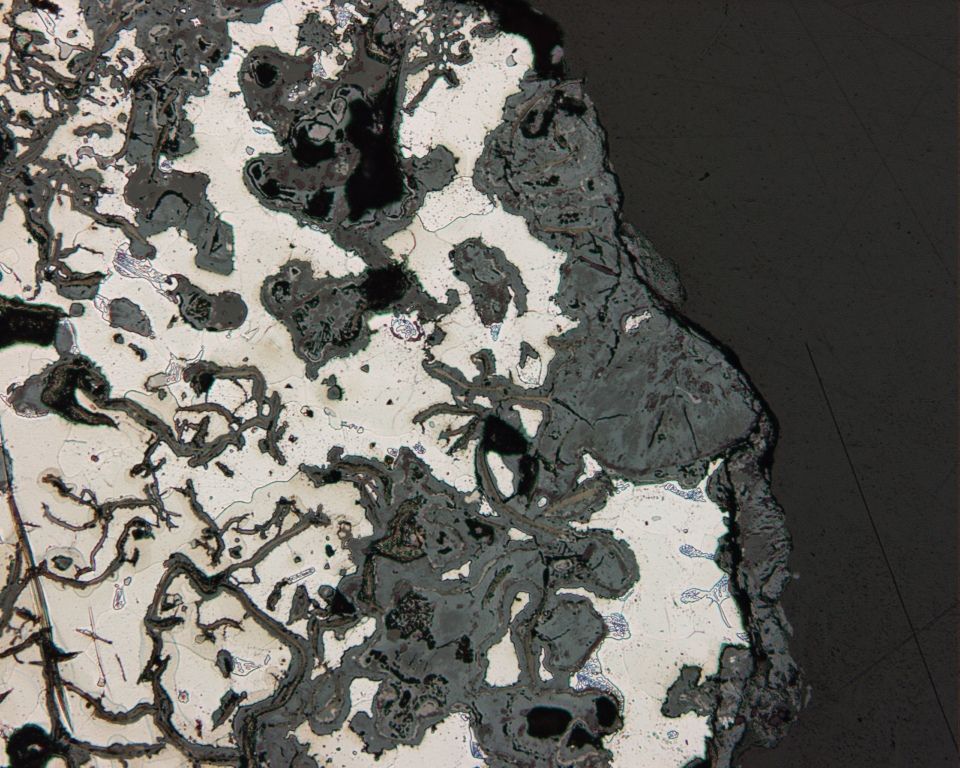
 Graphitic corrosion is selective leaching of
Graphitic corrosion is selective leaching of

Dezincification
Corrosion prevention Corrosion Nanotechnology
metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the ...
, selective leaching, also called dealloying, demetalification, parting and selective corrosion, is a corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
type in some solid solution
A solid solution, a term popularly used for metals, is a homogeneous mixture of two compounds in solid state and having a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solutio ...
alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s, when in suitable conditions a component of the alloys is preferentially leached from the initially homogenous material. The less noble
A noble is a member of the nobility.
Noble may also refer to:
Places Antarctica
* Noble Glacier, King George Island
* Noble Nunatak, Marie Byrd Land
* Noble Peak, Wiencke Island
* Noble Rocks, Graham Land
Australia
* Noble Island, Gr ...
metal is removed from the alloy by a microscopic-scale galvanic corrosion
Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, different metal, when both in the prese ...
mechanism. The most susceptible alloys are the ones containing metals with high distance between each other in the galvanic series
The galvanic series (or electropotential series) determines the nobility of metals and semi-metals. When two metals are submerged in an electrolyte, while also electrically connected by some external conductor, the less noble (base) will experien ...
, e.g. copper and zinc in brass. The elements most typically undergoing selective removal are zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
, aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
, chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
, and others.
Leaching of zinc
The most common example is selective leaching of zinc frombrass
Brass is an alloy of copper and zinc, in proportions which can be varied to achieve different colours and mechanical, electrical, acoustic and chemical properties, but copper typically has the larger proportion, generally copper and zinc. I ...
alloys containing more than 15% zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
(dezincification) in the presence of oxygen and moisture, e.g. from brass taps in chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
-containing water. Dezincification has been studied since the 1860s, and the mechanism by which it occurs was under extensive examination by the 1960s. It is believed that both copper and zinc gradually dissolve out simultaneously, and copper precipitates back from the solution. The material remaining is a copper-rich sponge with poor mechanical properties, and a color changed from yellow to red. Dezincification can be caused by water containing sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
, carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. Stagnant or low velocity waters tend to promote dezincification.
To combat this, arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
or tin
Tin is a chemical element; it has symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the ...
can be added to brass, or gunmetal
Gun metal, also known as red brass in the United States, is a type of bronze – an alloy of copper, tin, and zinc. Proportions vary but 88% copper, 8–10% tin, and 2–4% zinc is an approximation. Originally used chiefly for making cannon, ...
can be used instead. Dezincification resistant brass (DZR), also known as Brass C352 is an alloy used to make pipe fittings for use with potable water
Drinking water or potable water is water that is safe for ingestion, either when drunk directly in liquid form or consumed indirectly through food preparation. It is often (but not always) supplied through taps, in which case it is also calle ...
. Plumbing fittings that are resistant to dezincification are appropriately marked, with the letters "CR" (Corrosion Resistant) or DZR (dezincification resistant) in the UK, and the letters "DR" (dezincification resistant) in Australia.
Graphitic corrosion

 Graphitic corrosion is selective leaching of
Graphitic corrosion is selective leaching of iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, from grey cast iron
Cast iron is a class of iron–carbon alloys with a carbon content of more than 2% and silicon content around 1–3%. Its usefulness derives from its relatively low melting temperature. The alloying elements determine the form in which its car ...
, where iron is removed and graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
grains remain intact. Affected surfaces develop a layer of graphite, rust, and metallurgical impurities that may inhibit further leaching. The effect can be substantially reduced by alloying the cast iron with nickel.
Leaching of other elements
Dealuminification is a corresponding process for aluminum alloys. Similar effects for different metals aredecarburization
Decarburization (or decarbonization) is the process of decreasing carbon content, which is the opposite of carburization.
The term is typically used in metallurgy, describing the decrease of the content of carbon in metals (usually steel). Decar ...
(removal of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
from the surface of alloy), decobaltification, denickelification, etc. The prototypical system for dealloying to create nano-porous metals is the np-Au system, which is created by selectively leaching Ag out of an Au-Ag homogenous alloy.
Mechanisms
Liquid Metal Dealloying
When an initially homogenous alloy is placed in an acid that can preferentially dissolve one or more components out of the alloy, the remaining component will diffuse and organize into a unique, nano-porous microstructure. The resulting material will have ligaments, formed by the remaining material, surrounded by pores, empty space from which atoms were leached/diffused away.Porosity Development
The way that porosity develops during the dealloying process has been studied computationally to understand the diffusional pathways on an atomistic level. Firstly, the less noble atoms must be dissolved away from the surface of the alloy. This process is easiest for the lower coordinated atoms, i.e., those bonded to fewer other atoms, usually found as single atoms sitting on the surface ("adatoms"), but it is more difficult for higher coordinated atoms, i.e., those sitting at "steps" or in the bulk of the material. Thus, the slowest step, and that which is most important for determining rate of porosity evolution is the dissolution of these higher coordinated less noble atoms. Just as the less noble metal is less stable as an adatom on the surface, so is an atom of the more noble metal. Therefore, as dissolution proceeds, any more noble atoms will move to more stable positions, like steps, where its coordination is higher. This diffusion process is similar tospinodal decomposition
Spinodal decomposition is a mechanism by which a single thermodynamic Phase (matter), phase spontaneously separates into two phases (without nucleation). Decomposition occurs when there is no Thermodynamics, thermodynamic barrier to phase separatio ...
. Eventually, clusters of more noble atoms form this way, and surrounding less noble atoms dissolve away, leaving behind a "bicontinuous structure" and providing a pathway for dissolution to continue deeper into the metal.

Effects on Mechanical Properties
Testing Methods
Due to the relatively small sample size achievable with dealloying, the mechanical properties of these materials are often probed using the following techniques: * Nanoindentation * Micropillar compression * Deflection testing of bridges * Thin-film wrinklingStrength and Stiffness of Nano-porous Materials
A common concept in materials science is that, at ambient conditions, smaller features (like grain size or absolute size) generally lead to stronger materials (see Hall-Petch strengthening, Weibull statistics). However, due to the high-level of porosity in the dealloyed materials, their strengths and stiffnesses are relatively low compared to the bulk counterparts. The decrease in strength due to porosity can be described with the Gibson-Ashby (GA) relations, which give theyield strength
In materials science and engineering, the yield point is the point on a stress–strain curve that indicates the limit of elastic behavior and the beginning of plastic behavior. Below the yield point, a material will deform elastically and w ...
and Young's modulus
Young's modulus (or the Young modulus) is a mechanical property of solid materials that measures the tensile or compressive stiffness when the force is applied lengthwise. It is the modulus of elasticity for tension or axial compression. Youn ...
of a foam according to the following equations:
where and are geometric constants, and are microstructure dependent exponents, and is the relative density of the foam.
The GA relations can be used to estimate the strength and stiffness of a given dealloyed, porous material, but more extensive study has revealed an additional factor: ligament size. When the ligament diameter is greater than 100 nm, increasing ligament size leads to greater agreement between GA predictions and experimental measurements of yield stress and Young's modulus. However, when the ligament size is under 100 nm, which is very common in many dealloying processes, there is an addition to the GA strength that looks similar to Hall-Petch strengthening
In materials science, grain-boundary strengthening (or Hall–Petch strengthening) is a method of strengthening materials by changing their average crystallite (grain) size. It is based on the observation that grain boundaries are insurmountable ...
of bulk polycrystalline metals (i.e., the yield stress increases with the inverse square root of grain size). Combining this relationship with the GA relation from before, an expression for the yield stress of dealloyed materials with ligaments smaller than 100 nm can be determined:
where A and m are empirically determined constants, and is the ligament size. The represents the Hall-Petch-like contribution.
There are two theories for why this increase in strength occurs: 1) dislocations are less common in smaller sample volumes, so deformation requires activation of sources (which is a more difficult process), or 2) dislocations pile-up, which strengthens the material. Either way, there would be significant surface and small volume effects in the ligaments <100 nm, which lead to this increase in yield stress. A relationship between ligament size and Young's modulus has not been studied past the GA relation.
Occasionally, the metastable nature of these materials means that ligaments in the structure may "pinch off" due to surface diffusion, which decreases the connectivity of the structure, and reduces the strength of the dealloyed material past what would be expected from simply porosity (as predicted by the Gibson-Ashby relations).
Dislocation Motion in nano-porous materials
Because the ligaments of these materials are essentially small metallic samples, they are themselves expected to be quite ductile; although, the entire nano-porous material is often observed to be brittle in tension. Dislocation behavior is extensive within the ligaments (just as would be expected in a metal): a high density. of partial dislocations, stacking faults and twins have been observed both in simulation and in TEM. However, the morphology of the ligaments makes bulk dislocation motion very difficult; the limited size of each ligament and complex connectivity within the nano-porous structure means that a dislocation cannot freely travel long distances and thus induce large-scale plasticity.Countermeasures
Countermeasures involve using alloys not susceptible to grain boundary depletion, using a suitableheat treatment
Heat treating (or heat treatment) is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are a ...
, altering the environment (e.g. lowering oxygen content), and/or use cathodic protection
Cathodic protection (CP; ) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects the metal to be protected to a more easily corroded " sacrifi ...
.
Uses
Selective leaching can be used to produce powdered materials with extremely high surface area, such asRaney nickel
Raney nickel , also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable s ...
and other heterogeneous catalysts.{{Cite journal, last1=McCue, first1=Ian, last2=Benn, first2=Ellen, last3=Gaskey, first3=Bernard, last4=Erlebacher, first4=Jonah, date=2016-07-01, title=Dealloying and Dealloyed Materials, journal=Annual Review of Materials Research
The ''Annual Review of Materials Research'' is a peer-reviewed journal that publishes review articles about materials science. It has been published by the nonprofit Annual Reviews since 1971, when it was first released under the title the ''Annu ...
, volume=46, issue=1, pages=263–286, doi=10.1146/annurev-matsci-070115-031739, bibcode=2016AnRMS..46..263M, issn=1531-7331 Selective leaching can be the pre-final stage of depletion gilding.
See also
*Corrosion engineering
Corrosion engineering is an engineering specialty that applies scientific, technical, engineering skills, and knowledge of natural laws and physical resources to design and implement materials, structures, devices, systems, and procedures to mana ...
References
External links
Dezincification
Corrosion prevention Corrosion Nanotechnology