|
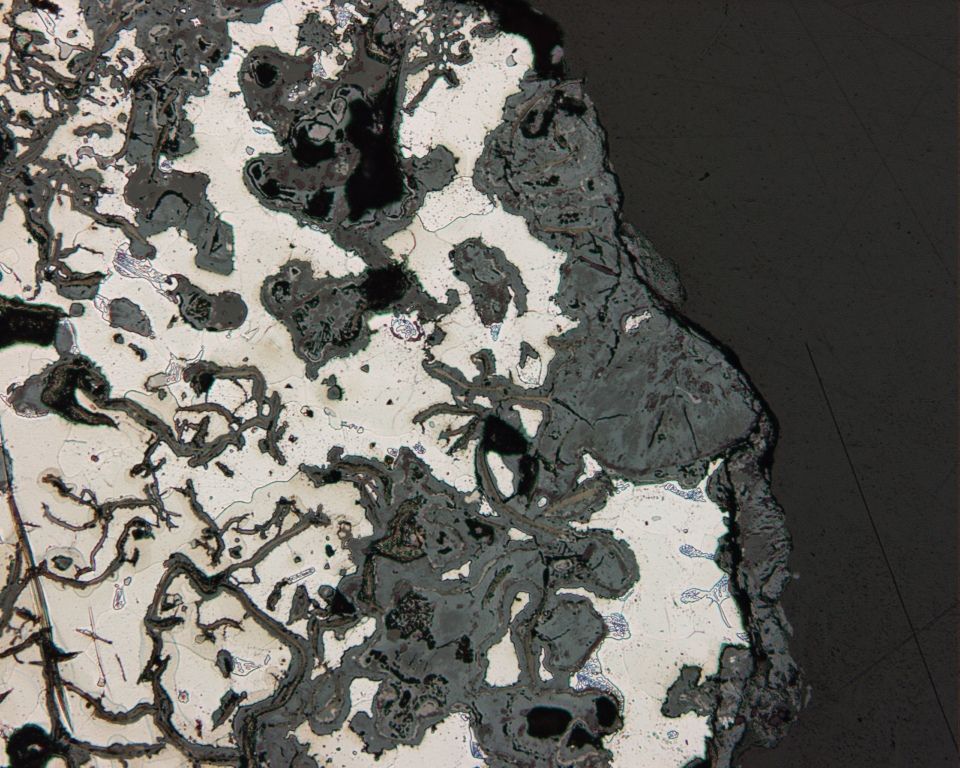
Selective Leaching
In metallurgy, selective leaching, also called dealloying, demetalification, parting and selective corrosion, is a corrosion type in some solid solution alloys, when in suitable conditions a component of the alloys is preferentially Leaching (metallurgy), leached from the initially homogenous material. The less noble metal, noble metal is removed from the alloy by a microscopic-scale galvanic corrosion mechanism. The most susceptible alloys are the ones containing metals with high distance between each other in the galvanic series, e.g. copper and zinc in brass. The elements most typically undergoing selective removal are zinc, aluminium, iron, cobalt, chromium, and others. Leaching of zinc The most common example is selective leaching of zinc from brass alloys containing more than 15% zinc (dezincification) in the presence of oxygen and moisture, e.g. from brass taps in chlorine-containing water. Dezincification has been studied since the 1860s, and the mechanism by which it oc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys. Metallurgy encompasses both the science and the technology of metals, including the production of metals and the engineering of metal components used in products for both consumers and manufacturers. Metallurgy is distinct from the craft of metalworking. Metalworking relies on metallurgy in a similar manner to how medicine relies on medical science for technical advancement. A specialist practitioner of metallurgy is known as a metallurgist. The science of metallurgy is further subdivided into two broad categories: chemical metallurgy and physical metallurgy. Chemical metallurgy is chiefly concerned with the reduction and oxidation of metals, and the chemical performance of metals. Subjects of study in chemical metallurgy include mineral processing, the extraction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
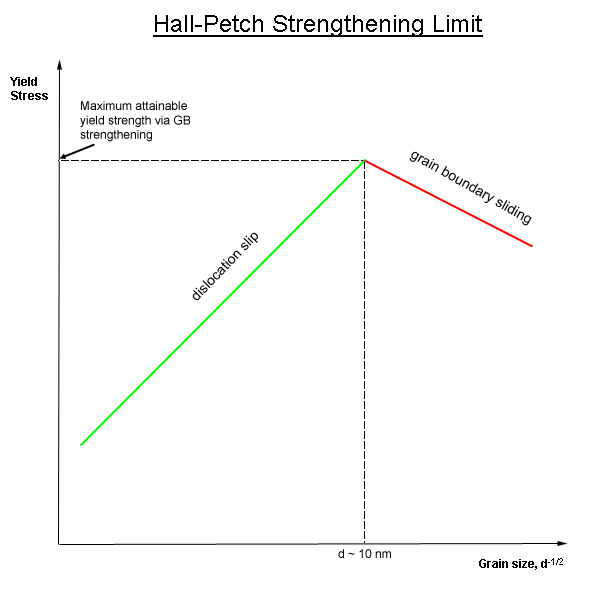
Hall-Petch
In materials science, grain-boundary strengthening (or Hall–Petch strengthening) is a method of strengthening materials by changing their average crystallite (grain) size. It is based on the observation that grain boundaries are insurmountable borders for dislocations and that the number of dislocations within a grain has an effect on how stress builds up in the adjacent grain, which will eventually activate dislocation sources and thus enabling deformation in the neighbouring grain as well. By changing grain size, one can influence the number of dislocations piled up at the grain boundary and yield strength. For example, heat treatment after plastic deformation and changing the rate of solidification are ways to alter grain size.W.D. Callister. Fundamentals of Materials Science and Engineering, 2nd ed. Wiley & Sons. pp. 252. Theory In grain-boundary strengthening, the grain boundaries act as pinning points impeding further dislocation propagation. Since the lattice structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
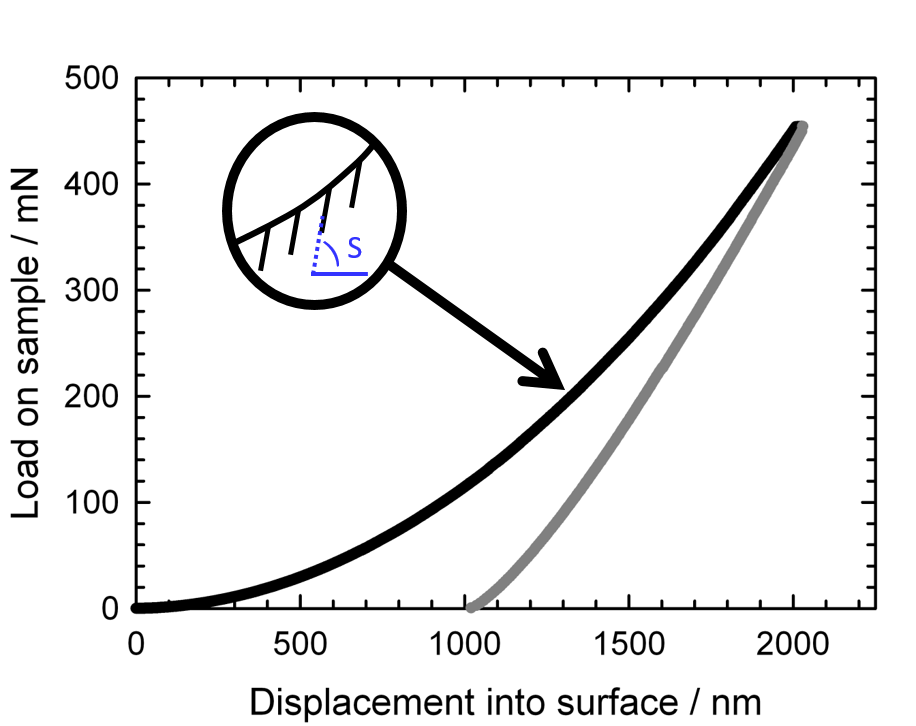
Nanoindentation
Nanoindentation, also called instrumented indentation testing, is a variety of indentation hardness tests applied to small volumes. Indentation is perhaps the most commonly applied means of testing the mechanical properties of materials. The nanoindentation technique was developed in the mid-1970s to measure the hardness of small volumes of material. Background In a traditional indentation test (macro or micro indentation), a hard tip whose mechanical properties are known (frequently made of a very hard material like diamond) is pressed into a sample whose properties are unknown. The load placed on the indenter tip is increased as the tip penetrates further into the specimen and soon reaches a user-defined value. At this point, the load may be held constant for a period or removed. The area of the residual indentation in the sample is measured and the hardness, H, is defined as the maximum load, P_\text, divided by the residual indentation area, A_\text: : H=\frac . For most te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spinodal Decomposition
Spinodal decomposition is a mechanism by which a single thermodynamic Phase (matter), phase spontaneously separates into two phases (without nucleation). Decomposition occurs when there is no Thermodynamics, thermodynamic barrier to phase separation. As a result, phase separation via decomposition does not require the nucleation events resulting from thermodynamic fluctuations, which normally trigger phase separation. Spinodal decomposition is observed when mixtures of metals or polymers separate into two co-existing phases, each rich in one species and poor in the other. When the two phases emerge in approximately equal proportion (each occupying about the same volume or area), characteristic intertwined structures are formed that gradually coarsen (see animation). The dynamics of spinodal decomposition is commonly modeled using the Cahn–Hilliard equation. Spinodal decomposition is fundamentally different from nucleation and growth. When there is a nucleation barrier to the form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarburization
Decarburization (or decarbonization) is the process of decreasing carbon content, which is the opposite of carburization. The term is typically used in metallurgy, describing the decrease of the content of carbon in metals (usually steel). Decarburization occurs when the metal is heated to temperatures of 700 °C or above when carbon in the metal reacts with gases containing oxygen or hydrogen. The removal of carbon removes hard carbide phases resulting in a softening of the metal, primarily at the surfaces which are in contact with the decarburizing gas. Decarburization can be either advantageous or detrimental, depending on the application for which the metal will be used. It is thus both something that can be done intentionally as a step in a manufacturing process, or something that happens as a side effect of a process (such as rolling) and must be either prevented or later reversed (such as via a carburization step). The decarburization mechanism can be described as th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cast Iron
Cast iron is a class of iron–carbon alloys with a carbon content of more than 2% and silicon content around 1–3%. Its usefulness derives from its relatively low melting temperature. The alloying elements determine the form in which its carbon appears: Cast iron#White cast iron, white cast iron has its carbon combined into an iron carbide named cementite, which is very hard, but brittle, as it allows cracks to pass straight through; Grey iron, grey cast iron has graphite flakes which deflect a passing crack and initiate countless new cracks as the material breaks, and Ductile iron, ductile cast iron has spherical graphite "nodules" which stop the crack from further progressing. Carbon (C), ranging from 1.8 to 4 wt%, and silicon (Si), 1–3 wt%, are the main alloying elements of cast iron. Iron alloys with lower carbon content are known as steel. Cast iron tends to be brittle, except for malleable iron, malleable cast irons. With its relatively low melting point, g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |