Salinity on:
[Wikipedia]
[Google]
[Amazon]
 Salinity () is the saltiness or amount of salt dissolved in a body of water, called
Salinity () is the saltiness or amount of salt dissolved in a body of water, called
Background papers and supporting data on the Practical Salinity Scale 1978
''Tech. Pap. Mar. Sci.'', 37 Salinities measured using PSS-78 do not have units. The suffix psu or PSU (denoting ''practical salinity unit'') is sometimes added to PSS-78 measurement values. The addition of PSU as a unit after the value is "formally incorrect and strongly discouraged". In 2010 a new standard for the properties of seawater called the ''thermodynamic equation of seawater 2010'' (
MIT page of seawater properties, with Matlab, EES and Excel VBA library routinesEquations and algorithms to calculate fundamental properties of sea water.History of the salinity determinationPractical Salinity Scale 1978.Salinity calculatorLewis, E. L. 1982. The practical salinity scale of 1978 and its antecedents. Marine Geodesy. 5(4):350–357.Equations and algorithms to calculate salinity of inland waters
{{Authority control Chemical oceanography Aquatic ecology Oceanography Coastal geography Water quality indicators Articles containing video clips Salts
 Salinity () is the saltiness or amount of salt dissolved in a body of water, called
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water
Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly sodium chloride). On the United States Geological Survey (USGS) salinity scale, saline water is saltier than brackish water, ...
(see also soil salinity
Soil salinity is the salt content in the soil; the process of increasing the salt content is known as salinization. Salts occur naturally within soils and water. Salination can be caused by natural processes such as mineral weathering or by the ...
). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to ‰).
Salinity is an important factor in determining many aspects of the chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
of natural waters and of biological
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary in ...
processes within it, and is a thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water.
A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''.
Definitions
Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds likesodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
, magnesium sulfate, potassium nitrate, and sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
which dissolve into ions. The concentration of dissolved chloride ions is sometimes referred to as chlorinity. Operationally, dissolved matter is defined as that which can pass through a very fine filter (historically a filter with a pore size of 0.45 μm, but nowadays usually 0.2 μm). Salinity can be expressed in the form of a mass fraction, i.e. the mass of the dissolved material in a unit mass of solution.
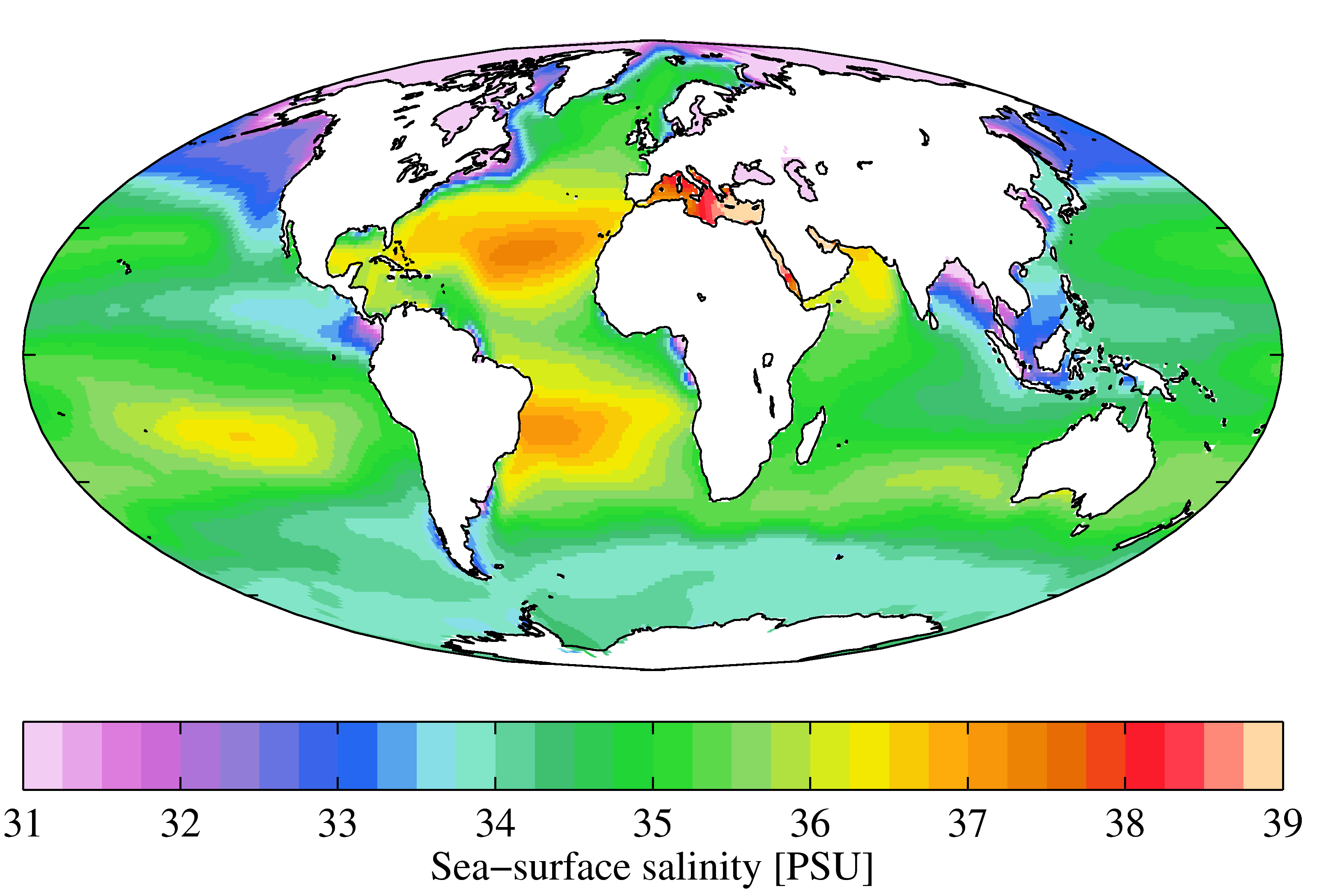
Seawater typically has a mass salinity of around 35 g/kg, although lower values are typical near coasts where rivers enter the ocean. Rivers and lakes can have a wide range of salinities, from less than 0.01 g/kg to a few g/kg, although there are many places where higher salinities are found. The Dead Sea
The Dead Sea ( he, יַם הַמֶּלַח, ''Yam hamMelaḥ''; ar, اَلْبَحْرُ الْمَيْتُ, ''Āl-Baḥrū l-Maytū''), also known by other names, is a salt lake bordered by Jordan to the east and Israel and the West Bank ...
has a salinity of more than 200 g/kg. Precipitation typically has a TDS of 20 mg/kg or less.
Whatever pore size is used in the definition, the resulting salinity value of a given sample of natural water will not vary by more than a few percent
In mathematics, a percentage (from la, per centum, "by a hundred") is a number or ratio expressed as a fraction of 100. It is often denoted using the percent sign, "%", although the abbreviations "pct.", "pct" and sometimes "pc" are also use ...
(%). Physical oceanographers working in the abyssal ocean, however, are often concerned with precision and intercomparability of measurements by different researchers, at different times, to almost five significant digits
Significant figures (also known as the significant digits, ''precision'' or ''resolution'') of a number in positional notation are digits in the number that are reliable and necessary to indicate the quantity of something.
If a number expre ...
. A bottled seawater product known as IAPSO Standard Seawater is used by oceanographers to standardize their measurements with enough precision to meet this requirement.
Composition
Measurement and definition difficulties arise because natural waters contain a complex mixture of many different elements from different sources (not all from dissolved salts) in different molecular forms. The chemical properties of some of these forms depend on temperature and pressure. Many of these forms are difficult to measure with high accuracy, and in any case complete chemical analysis is not practical when analyzing multiple samples. Different practical definitions of salinity result from different attempts to account for these problems, to different levels of precision, while still remaining reasonably easy to use. For practical reasons salinity is usually related to the sum of masses of a subset of these dissolved chemical constituents (so-called ''solution salinity''), rather than to the unknown mass of salts that gave rise to this composition (an exception is when artificial seawater is created). For many purposes this sum can be limited to a set of eight major ions in natural waters, although for seawater at highest precision an additional seven minor ions are also included. The major ions dominate the inorganic composition of most (but by no means all) natural waters. Exceptions include some pit lakes and waters from some hydrothermal springs. The concentrations of dissolved gases like oxygen and nitrogen are not usually included in descriptions of salinity. However, carbon dioxide gas, which when dissolved is partially converted into carbonates and bicarbonates, is often included. Silicon in the form of silicic acid, which usually appears as a neutral molecule in the pH range of most natural waters, may also be included for some purposes (e.g., when salinity/density relationships are being investigated).Seawater
The term 'salinity' is, for oceanographers, usually associated with one of a set of specific measurement techniques. As the dominant techniques evolve, so do different descriptions of salinity. Salinities were largely measured using titration-based techniques before the 1980s. Titration with silver nitrate could be used to determine the concentration ofhalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
ions (mainly chlorine and bromine) to give a chlorinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal ...
. The chlorinity was then multiplied by a factor to account for all other constituents. The resulting 'Knudsen salinities' are expressed in units of parts per thousand (ppt or ‰).
The use of electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
measurements to estimate the ionic content of seawater led to the development of the scale called the ''practical salinity scale 1978'' (PSS-78).Unesco (1981). The Practical Salinity Scale 1978 and the International Equation of State of Seawater 1980. ''Tech. Pap. Mar. Sci.'', 36Unesco (1981)Background papers and supporting data on the Practical Salinity Scale 1978
''Tech. Pap. Mar. Sci.'', 37 Salinities measured using PSS-78 do not have units. The suffix psu or PSU (denoting ''practical salinity unit'') is sometimes added to PSS-78 measurement values. The addition of PSU as a unit after the value is "formally incorrect and strongly discouraged". In 2010 a new standard for the properties of seawater called the ''thermodynamic equation of seawater 2010'' (
TEOS-10
TEOS-10 (Thermodynamic Equation of Seawater - 2010) is the international standard for the use and calculation of the thermodynamic properties of seawater, humid air and ice. It supersedes the former standard EOS-80 (Equation of State of Seawater 1 ...
) was introduced, advocating absolute salinity as a replacement for practical salinity, and conservative temperature
Conservative temperature (\Theta) is a thermodynamic property of seawater. It is derived from the potential enthalpy and is recommended under the TEOS-10 standard (Thermodynamic Equation of Seawater - 2010) as a replacement for potential temper ...
as a replacement for potential temperature
The potential temperature of a parcel of fluid at pressure P is the temperature that the parcel would attain if adiabatically brought to a standard reference pressure P_, usually . The potential temperature is denoted \theta and, for a gas well-a ...
. This standard includes a new scale called the ''reference composition salinity scale''. Absolute salinities on this scale are expressed as a mass fraction, in grams per kilogram of solution. Salinities on this scale are determined by combining electrical conductivity measurements with other information that can account for regional changes in the composition of seawater. They can also be determined by making direct density measurements.
A sample of seawater from most locations with a chlorinity of 19.37 ppt will have a Knudsen salinity of 35.00 ppt, a PSS-78 practical salinity of about 35.0, and a TEOS-10 absolute salinity of about 35.2 g/kg. The electrical conductivity of this water at a temperature of 15 °C is 42.9 mS/cm.
On the global scale, it is extremely likely that human-caused climate change has contributed to observed surface and subsurface salinity changes since the 1950s, and projections of surface salinity changes throughout the 21st century indicate that fresh ocean regions will continue to get fresher and salty regions will continue to get saltier.
Lakes and rivers
Limnologists and chemists often define salinity in terms of mass of salt per unit volume, expressed in units of mg/L or g/L. It is implied, although often not stated, that this value applies accurately only at some reference temperature because solution volume varies with temperature. Values presented in this way are typically accurate to the order of 1%. Limnologists also useelectrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
, or "reference conductivity", as a proxy for salinity. This measurement may be corrected for temperature effects, and is usually expressed in units of μS/cm.
A river or lake water with a salinity of around 70 mg/L will typically have a specific conductivity at 25 °C of between 80 and 130 μS/cm. The actual ratio depends on the ions present. The actual conductivity usually changes by about 2% per degree Celsius, so the measured conductivity at 5 °C might only be in the range of 50–80 μS/cm.
Direct density measurements are also used to estimate salinities, particularly in highly saline lakes. Sometimes density at a specific temperature is used as a proxy for salinity. At other times an empirical salinity/density relationship developed for a particular body of water is used to estimate the salinity of samples from a measured density.
Classification of water bodies based upon salinity
Marine waters are those of the ocean, another term for which is ''euhaline seas''. The salinity of euhaline seas is 30 to 35 ‰. ''Brackish seas'' or waters have salinity in the range of 0.5 to 29 ‰ and ''metahaline seas'' from 36 to 40 ‰. These waters are all regarded as ''thalassic'' because their salinity is derived from the ocean and defined as ''homoiohaline'' if salinity does not vary much over time (essentially constant). The table on the right, modified from Por (1972), follows the "Venice system" (1959). In contrast to homoiohaline environments are certain ''poikilohaline'' environments (which may also be ''thalassic'') in which the salinity variation is biologically significant. ''Poikilohaline'' water salinities may range anywhere from 0.5 to greater than 300 ‰. The important characteristic is that these waters tend to vary in salinity over some biologically meaningful range seasonally or on some other roughly comparable time scale. Put simply, these are bodies of water with quite variable salinity. Highly saline water, from which salts crystallize (or are about to), is referred to asbrine
Brine is a high-concentration solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of solutions used for br ...
.
Environmental considerations
Salinity is an ecological factor of considerable importance, influencing the types of organisms that live in a body of water. As well, salinity influences the kinds of plants that will grow either in a water body, or on land fed by a water (or by a groundwater). A plant adapted to saline conditions is called a halophyte. A halophyte which is tolerant to residual sodium carbonate salinity are calledglasswort
The glassworts are various succulent, annual halophytic plants, that is, plants that thrive in saline environments, such as seacoasts and salt marshes. The original English glasswort plants belong to the genus ''Salicornia'', but today the glass ...
or saltwort or barilla plants. Organisms (mostly bacteria) that can live in very salty conditions are classified as extremophiles, or halophile
The halophiles, named after the Greek word for "salt-loving", are extremophiles that thrive in high salt concentrations. While most halophiles are classified into the domain Archaea, there are also bacterial halophiles and some eukaryotic species, ...
s specifically. An organism that can withstand a wide range of salinities is euryhaline
Euryhaline organisms are able to adapt to a wide range of salinities. An example of a euryhaline fish is the molly (''Poecilia sphenops'') which can live in fresh water, brackish water, or salt water.
The green crab (''Carcinus maenas'') is an e ...
.
Salts are expensive to remove from water, and salt content is an important factor in water use, factoring into potability
Drinking water is water that is used in drink or food preparation; potable water is water that is safe to be used as drinking water. The amount of drinking water required to maintain good health varies, and depends on physical activity level, ag ...
and suitability for irrigation. Increases in salinity have been observed in lakes and rivers in the United States, due to common road salt and other salt de-icers in runoff.
The degree of salinity in oceans is a driver of the world's ocean circulation, where density changes due to both salinity changes and temperature changes at the surface of the ocean produce changes in buoyancy, which cause the sinking and rising of water masses. Changes in the salinity of the oceans are thought to contribute to global changes in carbon dioxide as more saline waters are less soluble to carbon dioxide. In addition, during glacial periods, the hydrography is such that a possible cause of reduced circulation is the production of stratified oceans. In such cases, it is more difficult to subduct water through the thermohaline circulation.
Not only is salinity a driver of ocean circulation, but changes in ocean circulation also affect salinity, particularly in the subpolar North Atlantic where from 1990 to 2010 increased contributions of Greenland meltwater were counteracted by increased northward transport of salty Atlantic waters. However, North Atlantic waters have become fresher since the mid-2010s due to increased Greenland meltwater flux.
See also
*Desalination for economic purposes **Desalination
Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in Soil salinity control, soil desalination, which is an issue f ...
of water
**Desalination of soil: soil salinity control
** Sodium adsorption ratio
* Measuring salinity
** Salinometer
* Salinity by biologic context
** In organisms generally, with particular emphasis on human health
*** Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
s
*** Fluid balance
*** Hypernatremia
*** Hyponatremia
*** Salt poisoning
Salt poisoning is an intoxication resulting from the excessive intake of sodium (usually as sodium chloride) in either solid form or in solution ( saline water, including brine, brackish water, eating salt, or seawater). Salt poisoning suffici ...
** In plants
*** ''Arabidopsis thaliana'' responses to salinity
** In fish
*** Stenohaline fish
*** Euryhaline fish
*Salinity by geologic context
**Fresh water
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salts and other total dissolved solids. Although the term specifically excludes seawater and brackish water, it does include ...
** Seawater
**Soil salinity
Soil salinity is the salt content in the soil; the process of increasing the salt content is known as salinization. Salts occur naturally within soils and water. Salination can be caused by natural processes such as mineral weathering or by the ...
** Thermohaline circulation
**Paleosalinity
Paleosalinity (or palaeosalinity) is the salinity of the global ocean or of an ocean basin at a point in geological history.
Importance
From Bjerrum plots, it is found that a decrease in the salinity of an aqueous fluid will act to increase th ...
** CORA dataset data on salinity of global oceans
*General cases of solute concentration
** Osmotic concentration
** Tonicity
References
Further reading
*MIT page of seawater properties, with Matlab, EES and Excel VBA library routines
{{Authority control Chemical oceanography Aquatic ecology Oceanography Coastal geography Water quality indicators Articles containing video clips Salts