ring-closing metathesis on:
[Wikipedia]
[Google]
[Amazon]
Ring-closing metathesis (RCM) is a widely used variation of  The most commonly synthesized ring sizes are between 5-7 atoms;Deiters, A.; Martin, S. F. (2004). “Synthesis of Oxygen- and Nitrogen-Containing Heterocycles by Ring-Closing Metathesis”. ''Chem. Rev.'' 104 (5): 2199-2238. . however, reported syntheses include 45- up to 90- membered macroheterocycles.Cain, M. F.; Forrest, W. P.; Peryshkov, R. V.; Schrock, R. R. Muller, P. (2013). “Synthesis of a TREN in Which the Aryl Substituents are Part of a 45 Atom Macrocycle”. ''J. Am. Chem. Soc.'' 135 (41): 15338-15341. .Dasgupta, S.; Wu, J. (2011). “Template-directed synthesis of kinetically and thermodynamically stable molecular necklace using ring closing metathesis”. ''Org. Biomol. Chem.'' 9: 3504-3515. Song, K. H.; Kang, S. O.; Ko, (2007). “Template Synthesis of a Huge Macrocycle by Olefin Metathesis Using Easily Accessible t(PEt3)2Templates”. ''Chem. Eur. J.'' 13 (18): 5129–5134. . These reactions are metal-catalyzed and proceed through a
The most commonly synthesized ring sizes are between 5-7 atoms;Deiters, A.; Martin, S. F. (2004). “Synthesis of Oxygen- and Nitrogen-Containing Heterocycles by Ring-Closing Metathesis”. ''Chem. Rev.'' 104 (5): 2199-2238. . however, reported syntheses include 45- up to 90- membered macroheterocycles.Cain, M. F.; Forrest, W. P.; Peryshkov, R. V.; Schrock, R. R. Muller, P. (2013). “Synthesis of a TREN in Which the Aryl Substituents are Part of a 45 Atom Macrocycle”. ''J. Am. Chem. Soc.'' 135 (41): 15338-15341. .Dasgupta, S.; Wu, J. (2011). “Template-directed synthesis of kinetically and thermodynamically stable molecular necklace using ring closing metathesis”. ''Org. Biomol. Chem.'' 9: 3504-3515. Song, K. H.; Kang, S. O.; Ko, (2007). “Template Synthesis of a Huge Macrocycle by Olefin Metathesis Using Easily Accessible t(PEt3)2Templates”. ''Chem. Eur. J.'' 13 (18): 5129–5134. . These reactions are metal-catalyzed and proceed through a
 In 1987, Siegfried Warwel and Hans Kaitker published a synthesis of symmetric macrocycles through a cross-metathesis dimerization of starting cycloolefins to afford C14, C18, and C20 dienes in 58-74% yield, as well as C16 in 30% yield, using Re2O7 on Al2O3 and Me4Sn for catalyst activation.
In 1987, Siegfried Warwel and Hans Kaitker published a synthesis of symmetric macrocycles through a cross-metathesis dimerization of starting cycloolefins to afford C14, C18, and C20 dienes in 58-74% yield, as well as C16 in 30% yield, using Re2O7 on Al2O3 and Me4Sn for catalyst activation.
 After a decade since its initial discovery, Grubbs and Fu published two influential reports in 1992 detailing the synthesis of O- and N- heterocycles via RCM utilizing Schrock’s molybdenum alkylidene catalysts, which had proven more robust and functional group tolerant than the tungsten chloride catalysts.Fu, G. C.; Grubbs, R. H. (1992). “The Application of Catalytic Ring-Closing Olefin Metathesis to the Synthesis of Unsaturated Oxygen Heterocycles”. ''J. Am. Chem. Soc.'' 114 (13): 5426-5427. .Fu, G. C.; Grubbs, R. H. (1992).“Synthesis of Nitrogen Heterocycles via Catalytic RingClosing Metathesis of Dienes”. ''J. Am. Chem. Soc.'' 114 (18): 7324-7325. . The synthetic route allowed access to
After a decade since its initial discovery, Grubbs and Fu published two influential reports in 1992 detailing the synthesis of O- and N- heterocycles via RCM utilizing Schrock’s molybdenum alkylidene catalysts, which had proven more robust and functional group tolerant than the tungsten chloride catalysts.Fu, G. C.; Grubbs, R. H. (1992). “The Application of Catalytic Ring-Closing Olefin Metathesis to the Synthesis of Unsaturated Oxygen Heterocycles”. ''J. Am. Chem. Soc.'' 114 (13): 5426-5427. .Fu, G. C.; Grubbs, R. H. (1992).“Synthesis of Nitrogen Heterocycles via Catalytic RingClosing Metathesis of Dienes”. ''J. Am. Chem. Soc.'' 114 (18): 7324-7325. . The synthetic route allowed access to  In 1993, Grubbs and others not only published a report on carbocycle synthesis using a molybdenum catalyst, but also detailed the initial use of a novel ruthenium carbene complex for metathesis reactions, which later became a popular catalyst due to its extraordinary utility. The ruthenium catalysts are not sensitive to air and moisture, unlike the molybdenum catalysts. The ruthenium catalysts, known better as the Grubbs Catalysts, as well as molybdenum catalysts, or Schrock’s Catalysts, are still used today for many metathesis reactions, including RCM. Overall, it was shown that metal-catalyzed RCM reactions were very effective in C-C bond forming reactions, and would prove of great importance in
In 1993, Grubbs and others not only published a report on carbocycle synthesis using a molybdenum catalyst, but also detailed the initial use of a novel ruthenium carbene complex for metathesis reactions, which later became a popular catalyst due to its extraordinary utility. The ruthenium catalysts are not sensitive to air and moisture, unlike the molybdenum catalysts. The ruthenium catalysts, known better as the Grubbs Catalysts, as well as molybdenum catalysts, or Schrock’s Catalysts, are still used today for many metathesis reactions, including RCM. Overall, it was shown that metal-catalyzed RCM reactions were very effective in C-C bond forming reactions, and would prove of great importance in
 Initiation occurs through substitution of the catalyst’s
Initiation occurs through substitution of the catalyst’s

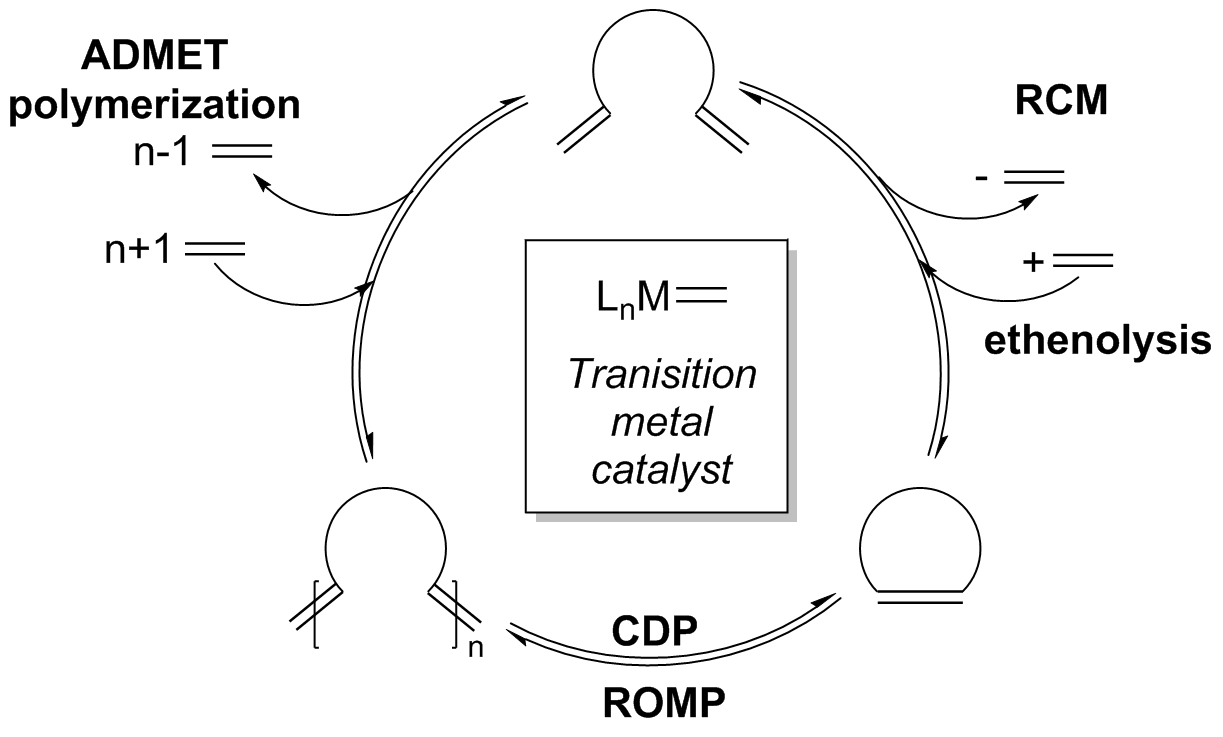 Although the reaction is still under thermodynamic control, an initial kinetic product, which may be dimerization or oligomerization of the starting material, is formed at the onset of the reaction as a result of higher catalyst reactivity. Increased catalyst activity also allows for the olefin products to reenter the catalytic cycle via non-terminal alkene addition onto the catalyst.Stewart, I. C.; Ung, T.; Pletnev, A. A.; Berlin, J. B.; Grubbs, R. H.; Schrodi, Y. (2007). “Highly Efficient Ruthenium Catalysts for the Formation of Tetrasubstituted Olefins via Ring-Closing Metathesis”. ''Org. Lett.'' 9 (8): 1589-1592. . Due to additional reactivity in strained olefins, an equilibrium distribution of products is observed; however, this equilibrium can be perturbed through a variety of techniques to overturn the product ratios in favor of the desired RCM product.
Although the reaction is still under thermodynamic control, an initial kinetic product, which may be dimerization or oligomerization of the starting material, is formed at the onset of the reaction as a result of higher catalyst reactivity. Increased catalyst activity also allows for the olefin products to reenter the catalytic cycle via non-terminal alkene addition onto the catalyst.Stewart, I. C.; Ung, T.; Pletnev, A. A.; Berlin, J. B.; Grubbs, R. H.; Schrodi, Y. (2007). “Highly Efficient Ruthenium Catalysts for the Formation of Tetrasubstituted Olefins via Ring-Closing Metathesis”. ''Org. Lett.'' 9 (8): 1589-1592. . Due to additional reactivity in strained olefins, an equilibrium distribution of products is observed; however, this equilibrium can be perturbed through a variety of techniques to overturn the product ratios in favor of the desired RCM product.
 Since the probability for reactive groups on the same molecule to encounter each other is inversely proportional to the ring size, the necessary intramolecular cycloaddition becomes increasingly difficult as ring size increases. This relationship means that the RCM of large rings is often performed under high dilution (0.05 - 100 mM) (A) to reduce
Since the probability for reactive groups on the same molecule to encounter each other is inversely proportional to the ring size, the necessary intramolecular cycloaddition becomes increasingly difficult as ring size increases. This relationship means that the RCM of large rings is often performed under high dilution (0.05 - 100 mM) (A) to reduce  Catalyst choice (D) has also been shown to be critical in controlling product formation. A few of the catalysts commonly used in ring-closing metathesis are shown below.
Catalyst choice (D) has also been shown to be critical in controlling product formation. A few of the catalysts commonly used in ring-closing metathesis are shown below.

 In addition to terminal
In addition to terminal 

 Another classic example is the use of a bulky
Another classic example is the use of a bulky  By orienting the molecule in such a way that the two reactive
By orienting the molecule in such a way that the two reactive
 Another common problem associated with RCM is the risk of catalyst degradation due to the high dilution required for some cyclizations. High dilution is also a limiting factor in industrial applications due to the large amount of waste generated from large-scale reactions at a low concentration. Efforts have been made to increase reaction concentration without compromising selectivity.
Another common problem associated with RCM is the risk of catalyst degradation due to the high dilution required for some cyclizations. High dilution is also a limiting factor in industrial applications due to the large amount of waste generated from large-scale reactions at a low concentration. Efforts have been made to increase reaction concentration without compromising selectivity.
 In 1995,
In 1995,  The ring strain in 8-11 atom rings has proven to be challenging for RCM; however, there are many cases where these cyclic systems have been synthesized. In 1997, Fürstner reported a facile synthesis to access jasmine ketolactone (''E/Z'') through a final RCM step. At the time, no previous 10-membered ring had been formed through RCM, and previous syntheses were often lengthy, involving a macrolactonization to form the decanolide. By adding the diene and catalyst over a 12-hour period to refluxing toluene, Fürstner was able to avoid oligomerization and obtain both ''E/Z'' isomers in 88% yield. CH2Cl2 favored the formation of the ''Z-''isomer in 1:2.5 (''E/Z'') ratio, whereas, toluene only afforded a 1:1.4 (''E/Z'') mixture.
The ring strain in 8-11 atom rings has proven to be challenging for RCM; however, there are many cases where these cyclic systems have been synthesized. In 1997, Fürstner reported a facile synthesis to access jasmine ketolactone (''E/Z'') through a final RCM step. At the time, no previous 10-membered ring had been formed through RCM, and previous syntheses were often lengthy, involving a macrolactonization to form the decanolide. By adding the diene and catalyst over a 12-hour period to refluxing toluene, Fürstner was able to avoid oligomerization and obtain both ''E/Z'' isomers in 88% yield. CH2Cl2 favored the formation of the ''Z-''isomer in 1:2.5 (''E/Z'') ratio, whereas, toluene only afforded a 1:1.4 (''E/Z'') mixture.
 In 2000, Alois Fürstner reported an eight step synthesis to access (−)-balanol using RCM to form a 7-member heterocycle intermediate. Balanol is a metabolite isolated from ''erticiullium balanoides'' and shows inhibitory action towards protein kinase C (PKC). In the ring closing metathesis step, a ruthenium indenylidene complex was used as the precatalyst to afford the desired 7-member ring in 87% yield.Furstner, A.; Thiel, O. R. (2000). “Formal Total Synthesis of (−)-Balanol: Concise Approach to the Hexahydroazepine Segment Based on RCM” . ''J. Org. Chem.'' 65 (6): 1738-1742. .
In 2000, Alois Fürstner reported an eight step synthesis to access (−)-balanol using RCM to form a 7-member heterocycle intermediate. Balanol is a metabolite isolated from ''erticiullium balanoides'' and shows inhibitory action towards protein kinase C (PKC). In the ring closing metathesis step, a ruthenium indenylidene complex was used as the precatalyst to afford the desired 7-member ring in 87% yield.Furstner, A.; Thiel, O. R. (2000). “Formal Total Synthesis of (−)-Balanol: Concise Approach to the Hexahydroazepine Segment Based on RCM” . ''J. Org. Chem.'' 65 (6): 1738-1742. .
 In 2002, Stephen F. Martin and others reported the 24-step synthesis of manzamine A with two ring-closing metathesis steps to access the polycyclic
In 2002, Stephen F. Martin and others reported the 24-step synthesis of manzamine A with two ring-closing metathesis steps to access the polycyclic  In 2003, Danishefsky and others reported the total synthesis of (+)-migrastatin, a
In 2003, Danishefsky and others reported the total synthesis of (+)-migrastatin, a  Overall, ring-closing metathesis is a highly useful reaction to readily obtain cyclic compounds of varying size and chemical makeup; however, it does have some limitations such as high dilution, selectivity, and unwanted isomerization.
Overall, ring-closing metathesis is a highly useful reaction to readily obtain cyclic compounds of varying size and chemical makeup; however, it does have some limitations such as high dilution, selectivity, and unwanted isomerization.
Ring-Closing Metathesis
at organic-chemistry.org
at sigmaaldrich.com
The Olefin Metathesis Reaction
Andrew Myers’ Group Notes {{DEFAULTSORT:Ring-Closing Metathesis Rearrangement reactions Organometallic chemistry Carbon-carbon bond forming reactions Homogeneous catalysis
olefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often create ...
in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s, which forms the cycloalkene as the ''E-'' or ''Z-'' isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
s and volatile ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
.Carey, F. A.; Sunburg, R. J. Reactions Involving Transition Metals. ''Advanced Organic Chemistry: Reaction and Synthesis'', 5th Ed.; Part B; Springer: New York, 2010, pp. 761-767.Monfette, S.; Fogg, D. E. (2009). "Equilibrium Ring-Closing Metathesis". ''Chem. Rev.'' 109 (8): 3783-3816. .
 The most commonly synthesized ring sizes are between 5-7 atoms;Deiters, A.; Martin, S. F. (2004). “Synthesis of Oxygen- and Nitrogen-Containing Heterocycles by Ring-Closing Metathesis”. ''Chem. Rev.'' 104 (5): 2199-2238. . however, reported syntheses include 45- up to 90- membered macroheterocycles.Cain, M. F.; Forrest, W. P.; Peryshkov, R. V.; Schrock, R. R. Muller, P. (2013). “Synthesis of a TREN in Which the Aryl Substituents are Part of a 45 Atom Macrocycle”. ''J. Am. Chem. Soc.'' 135 (41): 15338-15341. .Dasgupta, S.; Wu, J. (2011). “Template-directed synthesis of kinetically and thermodynamically stable molecular necklace using ring closing metathesis”. ''Org. Biomol. Chem.'' 9: 3504-3515. Song, K. H.; Kang, S. O.; Ko, (2007). “Template Synthesis of a Huge Macrocycle by Olefin Metathesis Using Easily Accessible t(PEt3)2Templates”. ''Chem. Eur. J.'' 13 (18): 5129–5134. . These reactions are metal-catalyzed and proceed through a
The most commonly synthesized ring sizes are between 5-7 atoms;Deiters, A.; Martin, S. F. (2004). “Synthesis of Oxygen- and Nitrogen-Containing Heterocycles by Ring-Closing Metathesis”. ''Chem. Rev.'' 104 (5): 2199-2238. . however, reported syntheses include 45- up to 90- membered macroheterocycles.Cain, M. F.; Forrest, W. P.; Peryshkov, R. V.; Schrock, R. R. Muller, P. (2013). “Synthesis of a TREN in Which the Aryl Substituents are Part of a 45 Atom Macrocycle”. ''J. Am. Chem. Soc.'' 135 (41): 15338-15341. .Dasgupta, S.; Wu, J. (2011). “Template-directed synthesis of kinetically and thermodynamically stable molecular necklace using ring closing metathesis”. ''Org. Biomol. Chem.'' 9: 3504-3515. Song, K. H.; Kang, S. O.; Ko, (2007). “Template Synthesis of a Huge Macrocycle by Olefin Metathesis Using Easily Accessible t(PEt3)2Templates”. ''Chem. Eur. J.'' 13 (18): 5129–5134. . These reactions are metal-catalyzed and proceed through a metallacyclobutane
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediate ...
intermediate.Schmalz, H.-G. (1995). “Catalytic Ring-Closing Metathesis : A New, Powerful Technique for Carbon- Carbon Coupling in Organic Synthesis”. ''Angew. Chem. Int. Ed. Engl.'' 34 (17): 1833-1836. . It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor,Villemin, D. (1980). “Synthese de Macrolides par Metathese”. ''Tetrahedron Lett.'' 21 (18): 1715-1718. . and later become popularized by Robert H. Grubbs
Robert Howard Grubbs ForMemRS (February 27, 1942 – December 19, 2021) was an American chemist and the Victor and Elizabeth Atkins Professor of Chemistry at the California Institute of Technology in Pasadena, California. He was a co-recipient ...
and Richard R. Schrock, who shared the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
, along with Yves Chauvin
Yves Chauvin (; 10 October 1930 – 27 January 2015) was a French chemist and Nobel Prize laureate. He was honorary research director at the ''Institut français du pétrole'' and a member of the French Academy of Science. He was known for his work ...
, in 2005 for their combined work in olefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often create ...
.Grubbs, R. H. (2006). “Olefin-Metathesis Catalysts for the Preparation of Molecules and Materials (Nobel Lecture)”. ''Angew. Chem. Int. Ed.'' 45 (23): 3760–3765. .Schrock, R. R. (2006). “Multiple Metal–Carbon Bonds for Catalytic Metathesis Reactions (Nobel Lecture)”. ''Angew. Chem. Int. Ed.'' 45 (23), 3748-3759. . RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope.Trnka, T. M.; Grubbs, R. H. (2001). “The Development of L2X2Ru=CHR Olefin Metathesis Catalysts: An Organometallic Success Story”. ''Acc. Chem. Res.'' 34 (1):18-29. . Since the only major by-product
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced.
A by-product can be useful and marketable or it can be consid ...
is ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry
Green chemistry, also called sustainable chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental che ...
.
There are several reviews published on ring-closing metathesis.Furstner, A. (2000). “Olefin Metathesis and Beyond”. ''Angew. Chem. Int. Ed.'' 39 (17): 3012-3043. .Gradillas, A.; Perez-Castells, J. (2006). “Macrocyclization by Ring-Closing Metathesis in the Total Synthesis of Natural Products: Reaction Conditions and Limitations”. ''Angew. Chem. Int. Ed.'' 45: 6086-6101. .
History
The first example of ring-closing metathesis was reported by Dider Villemin in 1980 when he synthesized an Exaltolide precursor using a WCl6/Me4Sn catalyzed metathesis cyclization in 60-65% yield depending on ring size (A). In the following months, Jiro Tsuji reported a similar metathesis reaction describing the preparation of a macrolide catalyzed by WCl6 and dimethyltitanocene (Cp2TiMe2) in a modest 17.9% yield (B). Tsuji describes theolefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often create ...
reaction as “…potentially useful in organic synthesis” and addresses the need for the development of a more versatile catalyst to tolerate various functional groups.
 In 1987, Siegfried Warwel and Hans Kaitker published a synthesis of symmetric macrocycles through a cross-metathesis dimerization of starting cycloolefins to afford C14, C18, and C20 dienes in 58-74% yield, as well as C16 in 30% yield, using Re2O7 on Al2O3 and Me4Sn for catalyst activation.
In 1987, Siegfried Warwel and Hans Kaitker published a synthesis of symmetric macrocycles through a cross-metathesis dimerization of starting cycloolefins to afford C14, C18, and C20 dienes in 58-74% yield, as well as C16 in 30% yield, using Re2O7 on Al2O3 and Me4Sn for catalyst activation.
 After a decade since its initial discovery, Grubbs and Fu published two influential reports in 1992 detailing the synthesis of O- and N- heterocycles via RCM utilizing Schrock’s molybdenum alkylidene catalysts, which had proven more robust and functional group tolerant than the tungsten chloride catalysts.Fu, G. C.; Grubbs, R. H. (1992). “The Application of Catalytic Ring-Closing Olefin Metathesis to the Synthesis of Unsaturated Oxygen Heterocycles”. ''J. Am. Chem. Soc.'' 114 (13): 5426-5427. .Fu, G. C.; Grubbs, R. H. (1992).“Synthesis of Nitrogen Heterocycles via Catalytic RingClosing Metathesis of Dienes”. ''J. Am. Chem. Soc.'' 114 (18): 7324-7325. . The synthetic route allowed access to
After a decade since its initial discovery, Grubbs and Fu published two influential reports in 1992 detailing the synthesis of O- and N- heterocycles via RCM utilizing Schrock’s molybdenum alkylidene catalysts, which had proven more robust and functional group tolerant than the tungsten chloride catalysts.Fu, G. C.; Grubbs, R. H. (1992). “The Application of Catalytic Ring-Closing Olefin Metathesis to the Synthesis of Unsaturated Oxygen Heterocycles”. ''J. Am. Chem. Soc.'' 114 (13): 5426-5427. .Fu, G. C.; Grubbs, R. H. (1992).“Synthesis of Nitrogen Heterocycles via Catalytic RingClosing Metathesis of Dienes”. ''J. Am. Chem. Soc.'' 114 (18): 7324-7325. . The synthetic route allowed access to dihydropyran In organic chemistry, dihydropyran refers to two heterocyclic compounds with the formula C5H8O:
* 3,4-Dihydro-2''H''-pyran
*3,6-dihydro-2''H''-pyran
Nomenclature
In IUPAC names, "dihydro" refers to the two added hydrogen atoms needed to remove o ...
s in high yield (89-93%) from readily available starting materials. In addition, synthesis of substituted pyrroline Pyrrolines, also known under the name dihydropyrroles, are three different heterocyclic organic chemical compounds that differ in the position of the double bond. Pyrrolines are formally derived from the aromate pyrrole by hydrogenation. 1-Pyrrolin ...
s, tetrahydropyridines, and amides
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
were illustrated in modest to high yield (73-89% ). The driving force for the cyclization reaction was attributed to entropic
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
favorability by forming two molecules per one molecule of starting material. The loss of the second molecule, ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
, a highly volatile gas, drives the reaction in the forward direction according to Le Châtelier's principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French ...
.
 In 1993, Grubbs and others not only published a report on carbocycle synthesis using a molybdenum catalyst, but also detailed the initial use of a novel ruthenium carbene complex for metathesis reactions, which later became a popular catalyst due to its extraordinary utility. The ruthenium catalysts are not sensitive to air and moisture, unlike the molybdenum catalysts. The ruthenium catalysts, known better as the Grubbs Catalysts, as well as molybdenum catalysts, or Schrock’s Catalysts, are still used today for many metathesis reactions, including RCM. Overall, it was shown that metal-catalyzed RCM reactions were very effective in C-C bond forming reactions, and would prove of great importance in
In 1993, Grubbs and others not only published a report on carbocycle synthesis using a molybdenum catalyst, but also detailed the initial use of a novel ruthenium carbene complex for metathesis reactions, which later became a popular catalyst due to its extraordinary utility. The ruthenium catalysts are not sensitive to air and moisture, unlike the molybdenum catalysts. The ruthenium catalysts, known better as the Grubbs Catalysts, as well as molybdenum catalysts, or Schrock’s Catalysts, are still used today for many metathesis reactions, including RCM. Overall, it was shown that metal-catalyzed RCM reactions were very effective in C-C bond forming reactions, and would prove of great importance in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, chemical biology
Chemical biology is a scientific discipline spanning the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and ma ...
, materials science, and various other fields to access a wide variety of unsaturated and highly functionalized cyclic analogues.
Mechanism
General mechanism
The mechanism fortransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
-catalyzed olefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often create ...
has been widely researched over the past forty years. RCM undergoes a similar mechanistic pathway as other olefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often create ...
reactions, such as cross metathesis (CM), ring-opening metathesis polymerization (ROMP), and acyclic diene metathesis (ADMET).Crabtree, R. H. Applications. ''The Organometallic Chemistry of the Transition Metals'', 6th Ed.; John Wiley & Sons, Inc.: New Jersey, 2014, pp.318-322. Since all steps in the catalytic cycle are considered reversible, it is possible for some of these other pathways to intersect with RCM depending on the reaction conditions and substrates. In 1971, Chauvin proposed the formation of a metallacyclobutane
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediate ...
intermediate through a +2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
which then cycloeliminates to either yield the same alkene and catalytic species (a nonproductive pathway), or produce a new catalytic species and an alkylidene
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been rep ...
(a productive pathway). This mechanism has become widely accepted among chemists and serves as the model for the RCM mechanism.Grossman, R. B. Transition-Metal-Catalyzed & -Mediated Reactions. ''The Art of Writing Reasonable Organic Reaction Mechanisms'', 2nd Ed.; Springer: New York, 2003, pp. 324-325.
 Initiation occurs through substitution of the catalyst’s
Initiation occurs through substitution of the catalyst’s alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
ligand with substrate. This process occurs via formation of a new alkylidene
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been rep ...
through one round of +2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
and cycloelimination. Association and dissociation of a phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligand also occurs in the case of Grubbs catalysts. In an RCM reaction, the alkylidene
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been rep ...
undergoes an intramolecular +2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
with the second reactive terminal alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
on the same molecule, rather than an intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
addition of a second molecule of starting material, a common competing side reaction which may lead to polymerization Cycloelimination of the metallacyclobutane
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediate ...
intermediate forms the desired RCM product along with a CH2, or alkylidene
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been rep ...
, species which reenters the catalytic cycle. While the loss of volatile ethylene is a driving force for RCM, it is also generated by competing metathesis reactions and therefore cannot be considered the only driving force of the reaction.
Thermodynamics
The reaction can be underkinetic
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory of gases, Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to i ...
or thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of ther ...
control depending on the exact reaction conditions, catalyst, and substrate. Common rings, 5- through 7-membered cycloalkenes, have a high tendency for formation and are often under greater thermodynamic control due to the enthalpic
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
favorability of the cyclic products, as shown by ''Illuminati'' and ''Mandolini'' on the formation of lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
rings.Illuminati, G.; Mandolini, L. (1981). “Ring Closure Reactions of Bifunctional Chain Molecules”. ''Acc. Chem. Res.'' 14 (5): 95-102. . Smaller rings, between 5 and 8 atoms, are more thermodynamically favored over medium to large rings due to lower ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are su ...
. Ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are su ...
arises from abnormal bond angles resulting in a higher heat of combustion
The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of heat released during the combustion of a specified amount of it.
The ''calorific value'' is the total energy relea ...
relative to the linear counterpart. If the RCM product contains a strained olefin, polymerization becomes preferable through ring-opening metathesis polymerization
Ring-opening metathesis polymerization (ROMP) is a type of olefin metathesis chain-growth polymerization. The driving force of the reaction is relief of ring strain in cyclic olefins (e.g. norbornene or cyclopentene). A variety of heterogeneous ...
of the newly formed olefin. Medium rings in particular have greater ring strain, in part due to greater transannular interactions from opposing sides of the ring, but also the inability to orient the molecule in such a way to prevent penalizing gauche interactions. RCM may be considered to have a kinetic bias if the products cannot reenter the catalytic cycle or interconvert through an equilibrium. A kinetic product distribution could lead to mostly RCM products or may lead to oligomers and polymers, which are most often disfavored.

Equilibrium
With the advent of more reactive catalysts, equilibrium RCM is observed quite often which may lead to a greater product distribution. The mechanism can be expanded to include the various competing equilibrium reactions as well as indicate where various side-products are formed along the reaction pathway, such as oligomers.Conrad, J. C.; Eelman, M. D.; Duarte Silva, J. A.; Monfette, S.; Parnas, H. H.; Snelgrove, J. L.; Fogg, D. E. (2007). “Oligomers as Intermediates in Ring-Closing Metathesis”. ''J. Am. Chem. Soc.'' 129 (5): 1024-1025. . Although the reaction is still under thermodynamic control, an initial kinetic product, which may be dimerization or oligomerization of the starting material, is formed at the onset of the reaction as a result of higher catalyst reactivity. Increased catalyst activity also allows for the olefin products to reenter the catalytic cycle via non-terminal alkene addition onto the catalyst.Stewart, I. C.; Ung, T.; Pletnev, A. A.; Berlin, J. B.; Grubbs, R. H.; Schrodi, Y. (2007). “Highly Efficient Ruthenium Catalysts for the Formation of Tetrasubstituted Olefins via Ring-Closing Metathesis”. ''Org. Lett.'' 9 (8): 1589-1592. . Due to additional reactivity in strained olefins, an equilibrium distribution of products is observed; however, this equilibrium can be perturbed through a variety of techniques to overturn the product ratios in favor of the desired RCM product.
Although the reaction is still under thermodynamic control, an initial kinetic product, which may be dimerization or oligomerization of the starting material, is formed at the onset of the reaction as a result of higher catalyst reactivity. Increased catalyst activity also allows for the olefin products to reenter the catalytic cycle via non-terminal alkene addition onto the catalyst.Stewart, I. C.; Ung, T.; Pletnev, A. A.; Berlin, J. B.; Grubbs, R. H.; Schrodi, Y. (2007). “Highly Efficient Ruthenium Catalysts for the Formation of Tetrasubstituted Olefins via Ring-Closing Metathesis”. ''Org. Lett.'' 9 (8): 1589-1592. . Due to additional reactivity in strained olefins, an equilibrium distribution of products is observed; however, this equilibrium can be perturbed through a variety of techniques to overturn the product ratios in favor of the desired RCM product.
 Since the probability for reactive groups on the same molecule to encounter each other is inversely proportional to the ring size, the necessary intramolecular cycloaddition becomes increasingly difficult as ring size increases. This relationship means that the RCM of large rings is often performed under high dilution (0.05 - 100 mM) (A) to reduce
Since the probability for reactive groups on the same molecule to encounter each other is inversely proportional to the ring size, the necessary intramolecular cycloaddition becomes increasingly difficult as ring size increases. This relationship means that the RCM of large rings is often performed under high dilution (0.05 - 100 mM) (A) to reduce intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
reactions; while the RCM of common rings can be performed at greater concentrations, even neat
Neat may refer to:
* Neat (bartending), a single, unmixed liquor served in a rocks glass
* Neat, an old term for horned oxen
* Neat Records, a British record label
* Neuroevolution of augmenting topologies (NEAT), a genetic algorithm (GA) for the ...
in rare cases. The equilibrium reaction can be driven to the desired thermodynamic products by increasing temperature (B), to decrease viscosity of the reaction mixture and therefore increase thermal motion, as well as increasing or decreasing reaction time (C).
 Catalyst choice (D) has also been shown to be critical in controlling product formation. A few of the catalysts commonly used in ring-closing metathesis are shown below.
Catalyst choice (D) has also been shown to be critical in controlling product formation. A few of the catalysts commonly used in ring-closing metathesis are shown below.

Reaction scope
Alkene substrate
Ring-closing Metathesis has shown utility in the synthesis of 5-30 membered rings, polycycles, and heterocycles containing atoms such as N, O, S, P, and even Si. Due to the functional group tolerance of modern RCM reactions, the synthesis of structurally complex compounds containing a range of functional groups such asepoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
s, ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s, alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s, ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
s, amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s, amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
s, and many others can be achieved more easily than previous methods. Oxygen and nitrogen heterocycles dominate due to their abundance in natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
s and pharmaceuticals. Some examples are shown below (the red alkene indicates C-C bond formed through RCM).
 In addition to terminal
In addition to terminal alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s, tri- and tetrasubstituted alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s have been used in RCM reactions to afford substituted cyclic olefin products. Ring-closing metathesis has also been used to cyclize rings containing an alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
to produce a new terminal alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
, or even undergo a second cyclization to form bicycles. This type of reaction is more formally known as enyne ring-closing metathesis.

''E''/''Z'' selectivity
In RCM reactions, two possible geometricisomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
s, either ''E-'' or ''Z-''isomer, may be formed. Stereoselectivity is dependent on the catalyst, ring strain, and starting diene. In smaller rings, ''Z-''isomers predominate as the more stable product reflecting ring-strain minimization. In macrocycles, the ''E-''isomer is often obtained as a result of the thermodynamic bias in RCM reactions as ''E-''isomers are more stable compared to ''Z-''isomers. As a general trend, ruthenium NHC (N-heterocyclic carbene) catalysts favor ''E'' selectivity to form the trans isomer. This in part due to the steric clash between the substituents, which adopt a trans configuration as the most stable conformation in the metallacyclobutane intermediate, to form the ''E-''isomer. The synthesis of stereopure ''Z-'' isomers were previously achieved via ring-closing alkyne metathesis. However, in 2013 Grubbs reported the use of a chelating ruthenium catalyst to afford ''Z'' macrocycles in high selectivity. The selectivity is attributed to the increased steric clash between the catalyst ligands and the metallacyclobutane intermediate that is formed. The increased steric interactions in the transition state lead to the ''Z'' olefin rather than the ''E'' olefin, because the transition state required to form the ''E-'' isomer is highly disfavored.

Cocatalyst
Additives are also used to overturn conformational preferences, increase reaction concentration, andchelate
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
highly polar groups, such as ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
s or amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
s, which can bind to the catalyst. Titanium isopropoxide (Ti(O''i''Pr)4) is commonly used to chelate
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
polar groups to prevent catalyst poisoning Catalyst poisoning refers to the partial or total deactivation of a catalyst by a chemical compound. Poisoning refers specifically to chemical deactivation, rather than other mechanisms of catalyst degradation such as thermal decomposition or physic ...
and in the case of an ester, the titanium Lewis acid binds the carbonyl oxygen. Once the oxygen is chelated with the titanium it can no longer bind to the ruthenium metal of the catalyst, which would result in catalyst deactivation. This also allows the reaction to be run at a higher effective concentration without dimerization of starting material.
 Another classic example is the use of a bulky
Another classic example is the use of a bulky Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
to form the ''E-''isomer of an ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
over the preferred ''Z''-isomer for cyclolactonization of medium rings. In one study, the addition of aluminum tris(2,6-diphenylphenoxide) (ATPH) was added to form a 7-membered lactone. The aluminum metal binds with the carbonyl oxygen forcing the bulky diphenylphenoxide groups in close proximity to the ester compound. As a result, the ester adopts the ''E-''isomer to minimize penalizing steric interactions. Without the Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, only the 14-membered dimer ring was observed.
 By orienting the molecule in such a way that the two reactive
By orienting the molecule in such a way that the two reactive alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s are in close proximity, the risk of intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
cross-metathesis is minimized.
Limitations
Many metathesis reactions with ruthenium catalysts are hampered by unwantedisomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
of the newly formed double bond, and it is believed that ruthenium hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
s that form as a side reaction are responsible. In one study it was found that isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
is suppressed in the RCM reaction of diallyl ether with specific additives capable of removing these hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
s. Without an additive, the reaction product is 2,3-dihydrofuran and not the expected 2,5-dihydrofuran
2,5-Dihydrofuran is an organic compound classified as a monounsaturated derivative of furan. It is a colorless, volatile liquid. It can be produced by the rearrangement of the epoxide of butadiene.G. Wytze Meindersma, Matthias Maase and André ...
(together with the formation of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
gas). Radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
scavengers, such as TEMPO
In musical terminology, tempo (Italian, 'time'; plural ''tempos'', or ''tempi'' from the Italian plural) is the speed or pace of a given piece. In classical music, tempo is typically indicated with an instruction at the start of a piece (often ...
or phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
, do not suppress isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
; however, additives such as 1,4-benzoquinone
1,4-Benzoquinone, commonly known as ''para''-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic o ...
or acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
successfully prevent unwanted isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
. Both additives are able to oxidize the ruthenium hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
s which may explain their behavior.
 Another common problem associated with RCM is the risk of catalyst degradation due to the high dilution required for some cyclizations. High dilution is also a limiting factor in industrial applications due to the large amount of waste generated from large-scale reactions at a low concentration. Efforts have been made to increase reaction concentration without compromising selectivity.
Another common problem associated with RCM is the risk of catalyst degradation due to the high dilution required for some cyclizations. High dilution is also a limiting factor in industrial applications due to the large amount of waste generated from large-scale reactions at a low concentration. Efforts have been made to increase reaction concentration without compromising selectivity.
Synthetic applications
Ring-closing metathesis has been used historically in numerousorganic syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and ex ...
and continues to be used today in the synthesis of a variety of compounds. The following examples are only representative of the broad utility of RCM, as there are numerous possibilities. For additional examples see the many review articles.
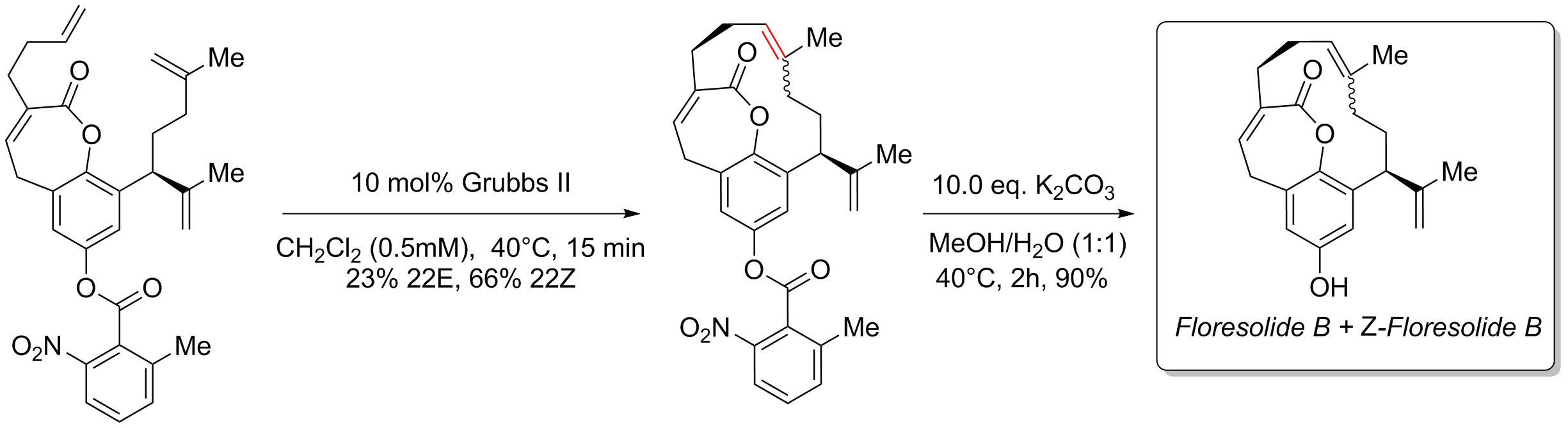
Ring-closing metathesis is important in total synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes ...
. One example is its use in the formation of the 12-membered ring in the synthesis of the naturally occurring cyclophane
In organic chemistry, a cyclophane is a hydrocarbon consisting of an aromatic unit (typically a benzene ring) and a chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic ...
floresolide. Floresolide B was isolated from an ascidian
Ascidiacea, commonly known as the ascidians, tunicates (in part), and sea squirts (in part), is a polyphyletic class in the subphylum Tunicata of sac-like marine invertebrate filter feeders. Ascidians are characterized by a tough outer "tunic" m ...
of the genus Apidium and showed cytotoxicity
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa'').
Cell physiology
Treating cells ...
against KB tumor cells. In 2005, K. C. Nicolaou
Kyriacos Costa Nicolaou ( el, Κυριάκος Κ. Νικολάου; born July 5, 1946) is a Cypriot-American chemist known for his research in the area of natural products total synthesis. He is currently Harry C. and Olga K. Wiess Professor of ...
and others completed a synthesis of both isomers through late-stage ring-closing metathesis using the 2nd Generation Grubbs catalyst to afford a mixture of ''E-'' and ''Z-'' isomers (1:3 ''E/Z'') in 89% yield. Although one prochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step. An achiral species which can be converted to a chiral in two steps is called proprochiral.
If two identical substituents are attach ...
center is present the product is racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
. Floresolide is an atropisomer
Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual co ...
as the new ring forms (due to steric constraints in the transition state) passing through the front of the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
group in and not the back. The carbonyl group then locks the ring permanently in place. The ''E/Z'' isomers were then separated and then the phenol nitrobenzoate protective group was removed in the final step by potassium carbonate to yield the final product and the unnatural ''Z''-isomer.
 In 1995,
In 1995, Robert Grubbs
Robert Howard Grubbs ForMemRS (February 27, 1942 – December 19, 2021) was an American chemist and the Victor and Elizabeth Atkins Professor of Chemistry at the California Institute of Technology in Pasadena, California. He was a co-recipient ...
and others highlighted the stereoselectivity possible with RCM. The group synthesized a diene with an internal hydrogen bond forming a β-turn. The hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
stabilized the macrocycle precursor placing both dienes in close proximity, primed for metathesis. After subjecting a mixture of diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
s to the reaction conditions, only one diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
of the olefin β-turn was obtained. The experiment was then repeated with (''S,S,S'') and (''R,S,R'') peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
s. Only the (''S,S,S'') diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
was reactive illustrating the configuration needed for ring-closing to be possible. The olefin product’s absolute configuration mimics that of Balaram’s disulfide peptide.
 The ring strain in 8-11 atom rings has proven to be challenging for RCM; however, there are many cases where these cyclic systems have been synthesized. In 1997, Fürstner reported a facile synthesis to access jasmine ketolactone (''E/Z'') through a final RCM step. At the time, no previous 10-membered ring had been formed through RCM, and previous syntheses were often lengthy, involving a macrolactonization to form the decanolide. By adding the diene and catalyst over a 12-hour period to refluxing toluene, Fürstner was able to avoid oligomerization and obtain both ''E/Z'' isomers in 88% yield. CH2Cl2 favored the formation of the ''Z-''isomer in 1:2.5 (''E/Z'') ratio, whereas, toluene only afforded a 1:1.4 (''E/Z'') mixture.
The ring strain in 8-11 atom rings has proven to be challenging for RCM; however, there are many cases where these cyclic systems have been synthesized. In 1997, Fürstner reported a facile synthesis to access jasmine ketolactone (''E/Z'') through a final RCM step. At the time, no previous 10-membered ring had been formed through RCM, and previous syntheses were often lengthy, involving a macrolactonization to form the decanolide. By adding the diene and catalyst over a 12-hour period to refluxing toluene, Fürstner was able to avoid oligomerization and obtain both ''E/Z'' isomers in 88% yield. CH2Cl2 favored the formation of the ''Z-''isomer in 1:2.5 (''E/Z'') ratio, whereas, toluene only afforded a 1:1.4 (''E/Z'') mixture.
 In 2000, Alois Fürstner reported an eight step synthesis to access (−)-balanol using RCM to form a 7-member heterocycle intermediate. Balanol is a metabolite isolated from ''erticiullium balanoides'' and shows inhibitory action towards protein kinase C (PKC). In the ring closing metathesis step, a ruthenium indenylidene complex was used as the precatalyst to afford the desired 7-member ring in 87% yield.Furstner, A.; Thiel, O. R. (2000). “Formal Total Synthesis of (−)-Balanol: Concise Approach to the Hexahydroazepine Segment Based on RCM” . ''J. Org. Chem.'' 65 (6): 1738-1742. .
In 2000, Alois Fürstner reported an eight step synthesis to access (−)-balanol using RCM to form a 7-member heterocycle intermediate. Balanol is a metabolite isolated from ''erticiullium balanoides'' and shows inhibitory action towards protein kinase C (PKC). In the ring closing metathesis step, a ruthenium indenylidene complex was used as the precatalyst to afford the desired 7-member ring in 87% yield.Furstner, A.; Thiel, O. R. (2000). “Formal Total Synthesis of (−)-Balanol: Concise Approach to the Hexahydroazepine Segment Based on RCM” . ''J. Org. Chem.'' 65 (6): 1738-1742. .
 In 2002, Stephen F. Martin and others reported the 24-step synthesis of manzamine A with two ring-closing metathesis steps to access the polycyclic
In 2002, Stephen F. Martin and others reported the 24-step synthesis of manzamine A with two ring-closing metathesis steps to access the polycyclic alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...
.Humphrey, J. H.; Liao, Y.; Ali, A.; Rein, T.; Wong, Y.-L.; Chen, H.-J.; Courtney, A. K.; Martin, S. F. (2002). “Enantioselective Total Syntheses of Manzamine A and Related Alkaloids”. ''J. Am. Chem. Soc.'' 124 (29): 8584-8592. . The natural product was isolated from marine sponges off the coast of Okinawa. Manzamine is a good target due to its potential as an antitumor compound. The first RCM step was to form the 13-member D ring as solely the ''Z''-isomer in 67% yield, a unique contrast to the usual favored ''E''-isomer of metathesis. After further transformations, the second RCM was used to form the 8-member E ring in 26% yield using stoichiometric 1st Generation Grubbs catalyst. The synthesis highlights the ability for functional group tolerance metathesis reactions as well as the ability to access complex molecules of varying ring sizes.
 In 2003, Danishefsky and others reported the total synthesis of (+)-migrastatin, a
In 2003, Danishefsky and others reported the total synthesis of (+)-migrastatin, a macrolide
The Macrolides are a class of natural products that consist of a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macrol ...
isolated from ''Streptomyces'' which inhibited tumor cell migration.Gaul, C.; Njardarson, J. T.; Danishefsky, S. J. (2003). “The Total Synthesis of (+)-Migrastatin”. ''J. Am. Chem. Soc.'' 125 (20): 6042-6043. . The macrolide
The Macrolides are a class of natural products that consist of a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macrol ...
contains a 14-member heterocycle that was formed through RCM. The metathesis reaction yielded the protected migrastatin
Migrastatin is an organic compound which naturally occurs in the '' Streptomyces platensis'' bacteria. Migrastatin and several of its analogues (including Isomigrastatin) have shown to have potential in treating cancer, as it inhibits the metastas ...
in 70% yield as only the (''E,E,Z'') isomer. It is reported that this selectivity arises from the preference for the ruthenium catalyst to add to the less hindered olefin first then cyclize to the most accessible olefin. The final deprotection of the silyl ether yielded (+)-migrastatin.
 Overall, ring-closing metathesis is a highly useful reaction to readily obtain cyclic compounds of varying size and chemical makeup; however, it does have some limitations such as high dilution, selectivity, and unwanted isomerization.
Overall, ring-closing metathesis is a highly useful reaction to readily obtain cyclic compounds of varying size and chemical makeup; however, it does have some limitations such as high dilution, selectivity, and unwanted isomerization.
See also
*Olefin Metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often create ...
* Ring-opening metathesis polymerization
Ring-opening metathesis polymerization (ROMP) is a type of olefin metathesis chain-growth polymerization. The driving force of the reaction is relief of ring strain in cyclic olefins (e.g. norbornene or cyclopentene). A variety of heterogeneous ...
* Alkane metathesis
* Alkyne metathesis
Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. T ...
* Enyne metathesis
An enyne metathesis is an organic reaction taking place between an alkyne and an alkene with a metal carbene catalyst forming a butadiene. This reaction is a variation of olefin metathesis.
The general scheme is given by ''scheme 1'':
:
When th ...
References
External links
Ring-Closing Metathesis
at organic-chemistry.org
at sigmaaldrich.com
The Olefin Metathesis Reaction
Andrew Myers’ Group Notes {{DEFAULTSORT:Ring-Closing Metathesis Rearrangement reactions Organometallic chemistry Carbon-carbon bond forming reactions Homogeneous catalysis