PRPP synthetase on:
[Wikipedia]
[Google]
[Amazon]
Ribose-phosphate diphosphokinase (or phosphoribosyl pyrophosphate synthetase or ribose-phosphate pyrophosphokinase) is an
 Ribose-phosphate diphosphokinase transfers the diphosphoryl group from Mg-ATP (Mg2+ coordinated to ATP) to ribose 5-phosphate. The enzymatic reaction begins with the binding of ribose 5-phosphate, followed by binding of Mg-ATP to the enzyme. In the transition state upon binding of both substrates, the diphosphate is transferred. The enzyme first releases AMP before releasing the product phosphoribosyl pyrophosphate.
Experiments using oxygen 18 labelled water demonstrate that the reaction mechanism proceeds with the nucleophilic attack of the anomeric hydroxyl group of ribose 5-phosphate on the beta-phosphorus of ATP in an
Ribose-phosphate diphosphokinase transfers the diphosphoryl group from Mg-ATP (Mg2+ coordinated to ATP) to ribose 5-phosphate. The enzymatic reaction begins with the binding of ribose 5-phosphate, followed by binding of Mg-ATP to the enzyme. In the transition state upon binding of both substrates, the diphosphate is transferred. The enzyme first releases AMP before releasing the product phosphoribosyl pyrophosphate.
Experiments using oxygen 18 labelled water demonstrate that the reaction mechanism proceeds with the nucleophilic attack of the anomeric hydroxyl group of ribose 5-phosphate on the beta-phosphorus of ATP in an 
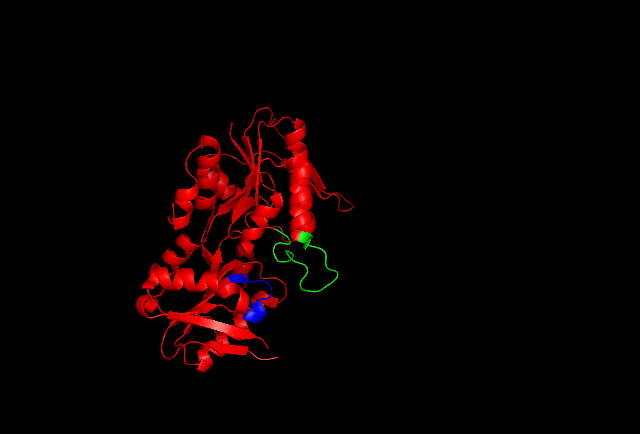
 Crystallization and X-ray diffraction studies elucidated the structure of the enzyme, which was isolated by cloning, protein expression, and purification techniques. One subunit of ribose-phosphate diphosphokinase consists of 318 amino acids; the active enzyme complex consists of three homodimers (or six subunits, a hexamer). The structure of one subunit is a five-stranded parallel
Crystallization and X-ray diffraction studies elucidated the structure of the enzyme, which was isolated by cloning, protein expression, and purification techniques. One subunit of ribose-phosphate diphosphokinase consists of 318 amino acids; the active enzyme complex consists of three homodimers (or six subunits, a hexamer). The structure of one subunit is a five-stranded parallel
Uniprot - Ribose-phosphate pyrophosphokinase 1
GeneReviews/NIH/NCBI/UW entry on Charcot-Marie-Tooth Neuropathy X Type 5
OMIM entries on Charcot-Marie-Tooth Neuropathy X Type 5
GeneReviews/NCBI/NIH/UW entry on Arts Syndrome
GeneReviews/NIH/NCBI/UW entry on Phosphoribosylpyrophosphate Synthetase (PRS) Superactivity
GeneReviews/NCBI/NIH/UW entry on DFNX1 Nonsyndromic Hearing Loss and Deafness
* {{Portal bar, Biology, border=no EC 2.7.6
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
that converts ribose 5-phosphate into phosphoribosyl pyrophosphate (PRPP). It is classified under .
The enzyme is involved in the synthesis of nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecu ...
s (purine
Purine is a heterocyclic aromatic organic compound that consists of two rings ( pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines ...
s and pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The othe ...
s), cofactors NAD and NADP, and amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the ...
and tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
, linking these biosynthetic processes to the pentose phosphate pathway, from which the substrate ribose 5-phosphate is derived. Ribose 5-phosphate is produced by the HMP Shunt Pathway from Glucose-6-Phosphate. The product phosphoribosyl pyrophosphate acts as an essential component of the purine salvage pathway and the de novo synthesis of purines. Dysfunction of the enzyme would thereby undermine purine metabolism
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.
Biosynthesis
Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ...
. Ribose-phosphate pyrophosphokinase exists in bacteria, plants, and animals, and there are three isoforms of human ribose-phosphate pyrophosphokinase. In humans, the genes encoding the enzyme are located on the X chromosome
The X chromosome is one of the two sex-determining chromosomes (allosomes) in many organisms, including mammals (the other is the Y chromosome), and is found in both males and females. It is a part of the XY sex-determination system and XO sex ...
.
Reaction mechanism
 Ribose-phosphate diphosphokinase transfers the diphosphoryl group from Mg-ATP (Mg2+ coordinated to ATP) to ribose 5-phosphate. The enzymatic reaction begins with the binding of ribose 5-phosphate, followed by binding of Mg-ATP to the enzyme. In the transition state upon binding of both substrates, the diphosphate is transferred. The enzyme first releases AMP before releasing the product phosphoribosyl pyrophosphate.
Experiments using oxygen 18 labelled water demonstrate that the reaction mechanism proceeds with the nucleophilic attack of the anomeric hydroxyl group of ribose 5-phosphate on the beta-phosphorus of ATP in an
Ribose-phosphate diphosphokinase transfers the diphosphoryl group from Mg-ATP (Mg2+ coordinated to ATP) to ribose 5-phosphate. The enzymatic reaction begins with the binding of ribose 5-phosphate, followed by binding of Mg-ATP to the enzyme. In the transition state upon binding of both substrates, the diphosphate is transferred. The enzyme first releases AMP before releasing the product phosphoribosyl pyrophosphate.
Experiments using oxygen 18 labelled water demonstrate that the reaction mechanism proceeds with the nucleophilic attack of the anomeric hydroxyl group of ribose 5-phosphate on the beta-phosphorus of ATP in an SN2 reaction
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed in a concerted way, i.e., in one step. The name SN2 refers to the Hughes-Ingold symbol of the m ...
.

Structure

 Crystallization and X-ray diffraction studies elucidated the structure of the enzyme, which was isolated by cloning, protein expression, and purification techniques. One subunit of ribose-phosphate diphosphokinase consists of 318 amino acids; the active enzyme complex consists of three homodimers (or six subunits, a hexamer). The structure of one subunit is a five-stranded parallel
Crystallization and X-ray diffraction studies elucidated the structure of the enzyme, which was isolated by cloning, protein expression, and purification techniques. One subunit of ribose-phosphate diphosphokinase consists of 318 amino acids; the active enzyme complex consists of three homodimers (or six subunits, a hexamer). The structure of one subunit is a five-stranded parallel beta sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a ge ...
(the central core) surrounded by four alpha helices
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues ear ...
at the N-terminal domain
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
and five alpha helices at the C-terminal domain
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain ( protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
, with two short anti-parallel beta-sheets extending from the core.
The catalytic site of the enzyme binds ATP and ribose 5-phosphate. The flexible loop (Phe92–Ser108), pyrophosphate binding loop (Asp171–Gly174), and flag region (Val30–Ile44 from an adjacent subunit) comprise the ATP binding site, located at the interface between two domains of one subunit. The flexible loop is so named because of its large variability in conformation. The ribose 5-phosphate binding site consists of residues Asp220–Thr228, located in the C-terminal domain of one subunit.
The allosteric site, which binds ADP, consists of amino acid residues from three subunits.
Function
The product of this reaction, phosphoribosyl pyrophosphate (PRPP), is used in numerous biosynthesis ( de novo and salvage) pathways. PRPP provides the ribose sugar in de novo synthesis of purines and pyrimidines, used in the nucleotide bases that form RNA and DNA. PRPP reacts withorotate
Orotic acid is a pyrimidinedione and a carboxylic acid. Historically, it was believed to be part of the vitamin B complex and was called vitamin B13, but it is now known that it is not a vitamin.
The compound is synthesized in the body via a m ...
to form orotidylate, which can be converted to uridylate (UMP). UMP can then be converted to the nucleotide cytidine triphosphate
Cytidine triphosphate (CTP) is a pyrimidine nucleoside triphosphate. CTP, much like ATP, consists of a ribose sugar, and three phosphate groups. The major difference between the two molecules is the base used, which in CTP is cytosine.
CTP is ...
(CTP). The reaction of PRPP, glutamine, and ammonia forms 5-Phosphoribosyl-1-amine, a precursor to inosinate (IMP), which can ultimately be converted to adenosine triphosphate (ATP) or guanosine triphosphate (GTP). PRPP plays a role in purine salvage pathways by reacting with free purine bases to form adenylate, guanylate, and inosinate. PRPP is also used in the synthesis of NAD: the reaction of PRPP with nicotinic acid yields the intermediate nicotinic acid mononucleotide.
Regulation
Ribose-phosphate diphosphokinase requires Mg2+ for activity; the enzyme acts only on ATP coordinated with Mg2+. Ribose-phosphate diphosphokinase is regulated by phosphorylation and allostery. It is activated byphosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
and inhibited by ADP
Adp or ADP may refer to:
Aviation
* Aéroports de Paris, airport authority for the Parisian region in France
* Aeropuertos del Perú, airport operator for airports in northern Peru
* SLAF Anuradhapura, an airport in Sri Lanka
* Ampara Airp ...
; it is suggested that phosphate and ADP compete for the same regulatory site. At normal concentrations, phosphate activates the enzyme by binding to its allosteric regulatory site. However, at high concentrations, phosphate is shown to have an inhibitory effect by competing with the substrate ribose 5-phosphate for binding at the active site. ADP is the key allosteric inhibitor of ribose-phosphate diphosphokinase. It has been shown that at lower concentrations of the substrate ribose 5-phosphate, ADP may inhibit the enzyme competitively. Ribose-phosphate pyrophosphokinase is also inhibited by some of its downstream biosynthetic products.
Role in disease
Because its product is a key compound in many biosynthetic pathways, ribose-phosphate diphosphokinase is involved in some rare disorders and X-linked recessive diseases. Mutations that lead to super-activity (increased enzyme activity or de-regulation of the enzyme) result in purine and uric acid overproduction. Super-activity symptoms includegout
Gout ( ) is a form of inflammatory arthritis characterized by recurrent attacks of a red, tender, hot and swollen joint, caused by deposition of monosodium urate monohydrate crystals. Pain typically comes on rapidly, reaching maximal intens ...
, sensorineural hearing loss, weak muscle tone (hypotonia), impaired muscle coordination (ataxia), hereditary peripheral neuropathy, and neurodevelopmental disorder.
Mutations that lead to loss-of-function in ribose-phosphate diphosphokinase result in Charcot-Marie-Tooth disease and Arts syndrome.
References
External links
Uniprot - Ribose-phosphate pyrophosphokinase 1
GeneReviews/NIH/NCBI/UW entry on Charcot-Marie-Tooth Neuropathy X Type 5
OMIM entries on Charcot-Marie-Tooth Neuropathy X Type 5
GeneReviews/NCBI/NIH/UW entry on Arts Syndrome
GeneReviews/NIH/NCBI/UW entry on Phosphoribosylpyrophosphate Synthetase (PRS) Superactivity
GeneReviews/NCBI/NIH/UW entry on DFNX1 Nonsyndromic Hearing Loss and Deafness
* {{Portal bar, Biology, border=no EC 2.7.6