Phenylpropanoid Metabolic Pathway on:
[Wikipedia]
[Google]
[Amazon]
The
biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. ...
of phenylpropanoid
The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of ...
s involves a number of enzymes.
From amino acids to cinnamates
In plants, all phenylpropanoids are derived from the amino acidsphenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino a ...
and tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Gr ...
.
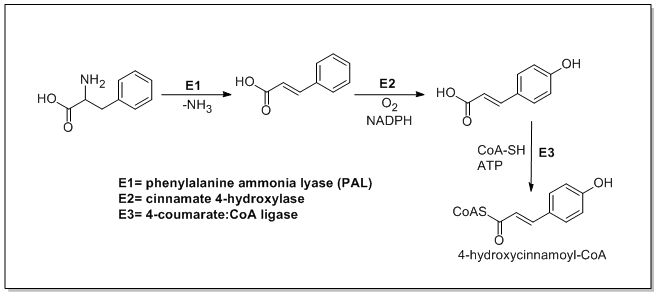
Phenylalanine ammonia-lyase
The enzyme phenylalanine ammonia lyase (EC 4.3.1.24) catalyzes the conversion of L-phenylalanine to ammonia and ''trans''-cinnamic acid.:
:L-phenylalanine = ''trans''-cinnamate + NH3
Phenylalanine ammonia lyase (PAL) is the first and committed ...
(PAL, a.k.a. phenylalanine/tyrosine ammonia-lyase) is an enzyme that transforms L-phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino a ...
and tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Gr ...
into trans-cinnamic acid
Cinnamic acid is an organic compound with the formula C6H5-CH=CH- COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs n ...
and ''p''-coumaric acid, respectively.
Trans-cinnamate 4-monooxygenase
In enzymology, a trans-cinnamate 4-monooxygenase () is an enzyme that catalyzes the chemical reaction
:trans-cinnamate + NADPH + H+ + O2 \rightleftharpoons 4-hydroxycinnamate + NADP+ + H2O
The 4 substrates of this enzyme are trans-cinnamate, N ...
(cinnamate 4-hydroxylase) is the enzyme that transforms trans-cinnamate into 4-hydroxycinnamate (''p''-coumaric acid). 4-Coumarate-CoA ligase is the enzyme that transforms 4-coumarate (''p''-coumaric acid) into 4-coumaroyl-CoA
Coumaroyl-coenzyme A is the thioester of coenzyme-A and coumaric acid. Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include Monolignol, lignols (precursors to lignin ...
.

Enzymes associated with biosynthesis of hydroxycinnamic acids
*Cinnamyl-alcohol dehydrogenase
In enzymology, a cinnamyl-alcohol dehydrogenase () is an enzyme that catalyzes the chemical reaction
:cinnamyl alcohol + NADP+ \rightleftharpoons cinnamaldehyde + NADPH + H+
Thus, the two substrates of this enzyme are cinnamyl alcohol and NADP+ ...
(CAD), an enzyme that transforms cinnamyl alcohol
Cinnamyl alcohol or styron is an organic compound that is found in esterified form in storax, Balsam of Peru, and cinnamon leaves. It forms a white crystalline solid when pure, or a yellow oil when even slightly impure. It can be produced by the h ...
into cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula(C9H8O) C6H5CH=CHCHO. Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shik ...
* Sinapine esterase
The enzyme sinapine esterase (EC 3.1.1.49) catalyzes the reaction
:sinapoylcholine + H2O \rightleftharpoons sinapate + choline
This enzyme belongs to the family of hydrolases, specifically those acting on carboxylic ester bonds. The systematic ...
, an enzyme that transforms sinapoylcholine
Sinapine is an alkaloidal amine found in some seeds, particularly oil seeds of plants in the family Brassicaceae. It is the choline ester of sinapic acid.
Sinapine was discovered by Etienne Ossian Henry in 1825.
Occurrence
Sinapine typically oc ...
into sinapate (sinapic acid
Sinapinic acid, or sinapic acid (Sinapine - Origin: L. Sinapi, sinapis, mustard, Gr., cf. F. Sinapine.), is a small naturally occurring hydroxycinnamic acid. It is a member of the phenylpropanoid family. It is a commonly used matrix in MALDI mass ...
) and choline Choline is an essential nutrient for humans and many other animals. Choline occurs as a cation that forms various salts (X− in the depicted formula is an undefined counteranion). Humans are capable of some ''de novo synthesis'' of choline but re ...
* Trans-cinnamate 2-monooxygenase
In enzymology, a trans-cinnamate 2-monooxygenase () is an enzyme that catalyzes the chemical reaction
:trans-cinnamate + NADPH + H+ + O2 \rightleftharpoons 2-hydroxycinnamate + NADP+ + H2O
The 4 substrates of this enzyme are trans-cinnamate, ...
, an enzyme that transforms trans-cinnamate (cinnamic acid
Cinnamic acid is an organic compound with the formula C6H5-CH=CH- COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs n ...
) into 2-hydroxycinnamate
''o''-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers of coumaric acids — ''o''-coumaric acid, ''m''-coumaric acid, and ''p''-coumaric acid — that differ by ...
* Caffeate O-methyltransferase
In enzymology, a caffeate ''O''-methyltransferase () is an enzyme that catalyzes the chemical reaction
:''S''-adenosyl-L-methionine + 3,4-dihydroxy-''trans''-cinnamate \rightleftharpoons ''S''-adenosyl-L-homocysteine + 3-methoxy-4-hydroxy-''trans ...
, an enzyme that transforms caffeic acid
Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one ...
into ferulic acid
Ferulic acid is a hydroxycinnamic acid, an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus ''Ferula'', referring to the giant fennel (''Ferula communis''). Classified as a phenolic phytochemical, ferulic ...
* Caffeoyl-CoA O-methyltransferase
In enzymology, a caffeoyl-CoA O-methyltransferase () is an enzyme that catalyzes the chemical reaction
:S-adenosyl-L-methionine + caffeoyl-CoA \rightleftharpoons S-adenosyl-L-homocysteine + feruloyl-CoA
Thus, the two substrates of this enzyme a ...
, an enzyme that transforms caffeoyl-CoA into feruloyl-CoA
* 5-O-(4-coumaroyl)-D-quinate 3'-monooxygenase
In enzymology, a 5-O-(4-coumaroyl)-D-quinate 3'-monooxygenase () is an enzyme that catalyzes the chemical reaction
:trans-5-O-(4-coumaroyl)-D-quinate + NADPH + H+ + O2 \rightleftharpoons trans-5-O-caffeoyl-D-quinate + NADP+ + H2O
The 4 substra ...
, an enzyme that transforms trans-5-O-(4-coumaroyl)-D-quinate into trans-5-O-caffeoyl-D-quinate
* Sinapoylglucose—choline O-sinapoyltransferase
In enzymology, a sinapoylglucose---choline O-sinapoyltransferase () is an enzyme that catalysis, catalyzes the chemical reaction
:1-O-sinapoyl-beta-D-glucose + choline \rightleftharpoons D-glucose + sinapoylcholine
Thus, the two substrate (bioche ...
, an enzyme that transforms 1-O-sinapoyl-beta-D-glucose into sinapoylcholine (sinapine
Sinapine is an alkaloidal amine found in some seeds, particularly oil seeds of plants in the family Brassicaceae. It is the choline ester of sinapic acid.
Sinapine was discovered by Etienne Ossian Henry in 1825.
Occurrence
Sinapine typically oc ...
)
* Sinapoylglucose—malate O-sinapoyltransferase, an enzyme that transforms 1-O-sinapoyl-beta-D-glucose into sinapoyl-(S)-malate
* Cinnamoyl-CoA reductase
Cinnamoyl-CoA reductase (), systematically named cinnamaldehyde:NADP+ oxidoreductase (CoA-cinnamoylating) but commonly referred to by the acronym CCR, is an enzyme that catalyzes the reduction of a substituted cinnamoyl-CoA to its corresponding ...
, an enzyme that transforms cinnamoyl-CoA
Cinnamoyl-Coenzyme A is an intermediate in the phenylpropanoids metabolic pathway.
Enzymes using Cinnamoyl-Coenzyme A
* Cinnamoyl-CoA reductase, an enzyme that catalyzes the chemical reaction cinnamaldehyde + CoA + NADP+ → cinnamoyl-CoA + NADP ...
from cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula(C9H8O) C6H5CH=CHCHO. Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shik ...
Conjugation enzymes
These enzymes conjugate phenylpropanoids to other molecules. * 2-coumarate O-beta-glucosyltransferase, the enzyme that transforms trans-2-hydroxycinnamate into trans-beta-D-glucosyl-2-hydroxycinnamate * Hydroxycinnamate 4-beta-glucosyltransferase, the enzyme that transforms p-coumaric acid into 4-O-beta-D-glucosyl-4-hydroxycinnamate *Shikimate O-hydroxycinnamoyltransferase
In enzymology, a shikimate O-hydroxycinnamoyltransferase () is an enzyme that catalyzes the chemical reaction
:4-coumaroyl-CoA + shikimate \rightleftharpoons CoA + 4-coumaroylshikimate
Thus, the two substrates of this enzyme are 4-coumaroyl-CoA ...
, the enzyme that transforms 4-coumaroyl-CoA
Coumaroyl-coenzyme A is the thioester of coenzyme-A and coumaric acid. Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include Monolignol, lignols (precursors to lignin ...
into 4-coumaroylshikimate
* Quinate O-hydroxycinnamoyltransferase, the enzyme that transforms feruloyl-CoA into O-feruloylquinate
* Sinapate 1-glucosyltransferase, the enzyme that transforms sinapate (sinapic acid
Sinapinic acid, or sinapic acid (Sinapine - Origin: L. Sinapi, sinapis, mustard, Gr., cf. F. Sinapine.), is a small naturally occurring hydroxycinnamic acid. It is a member of the phenylpropanoid family. It is a commonly used matrix in MALDI mass ...
) into 1-sinapoyl-D-glucose
* Coniferyl-alcohol glucosyltransferase
In enzymology, a coniferyl-alcohol glucosyltransferase () is an enzyme that catalyzes the chemical reaction
:UDP-glucose + coniferyl alcohol \rightleftharpoons UDP + coniferin
Thus, the two substrates of this enzyme are UDP-glucose and conifery ...
, the enzyme that transforms coniferyl alcohol
Coniferyl alcohol is an organic compound with the formula HO(CH3O)C6H3CH=CHCH2OH. A colourless or white solid, it is one of the monolignols, produced via the phenylpropanoid biochemical pathway. When copolymerized with related aromatic compounds, ...
into coniferin
Coniferin is a glucoside of coniferyl alcohol. This white crystalline solid is a metabolite in conifers, serving as an intermediate in cell wall lignification, as well as having other biological roles. It can also be found in the water root extrac ...
Deconjugation enzymes
* Coniferin beta-glucosidase, in theglucosidase
Glucosidases are the glycoside hydrolase enzymes categorized under the EC number 3.2.1.
Function
Alpha-glucosidases are enzymes involved in breaking down complex carbohydrates such as starch and glycogen into their monomers.
They catalyze t ...
that transforms coniferin
Coniferin is a glucoside of coniferyl alcohol. This white crystalline solid is a metabolite in conifers, serving as an intermediate in cell wall lignification, as well as having other biological roles. It can also be found in the water root extrac ...
into coniferol
Stilbenoids biosynthesis
*Pinosylvin synthase
In enzymology, a pinosylvin synthase () is an enzyme that catalyzes the chemical reaction
:3 malonyl-CoA + cinnamoyl-CoA \rightleftharpoons 4 CoA + pinosylvin + 4 CO2
Thus, the two substrates of this enzyme are malonyl-CoA and cinnamoyl-CoA, ...
, an enzyme that transforms pinosylvin
Pinosylvin is an organic compound with the formula C6H5CH=CHC6H3(OH)2. A white solid, it is related to trans-stilbene, but with two hydroxy groups on one of the phenyl substituents. It is very soluble in many organic solvents, such as acetone.
O ...
from cinnamoyl-CoA
Cinnamoyl-Coenzyme A is an intermediate in the phenylpropanoids metabolic pathway.
Enzymes using Cinnamoyl-Coenzyme A
* Cinnamoyl-CoA reductase, an enzyme that catalyzes the chemical reaction cinnamaldehyde + CoA + NADP+ → cinnamoyl-CoA + NADP ...
* Trihydroxystilbene synthase
In enzymology, a trihydroxystilbene synthase () is an enzyme that catalyzes the chemical reaction
:3 malonyl-CoA + 4-coumaroyl-CoA \rightleftharpoons 4 CoA + 3,4',5-trihydroxy-stilbene + 4 CO2
Thus, the two substrates of this enzyme are malonyl- ...
, an enzyme that transforms 4-coumaroyl-CoA
Coumaroyl-coenzyme A is the thioester of coenzyme-A and coumaric acid. Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include Monolignol, lignols (precursors to lignin ...
to resveratrol
Resveratrol (3,5,4′-trihydroxy-''trans''-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacteria or fungi. Sources ...
.
An alternative bacterial ketosynthase Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the result ...
-directed stilbenoid
Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are prod ...
s biosynthesis pathway exists in ''Photorhabdus
''Photorhabdus'' is a genus of bioluminescent, gram-negative bacilli which lives symbiotically within entomopathogenic nematodes, hence the name ''photo'' (which means light producing) and ''rhabdus'' (rod shape). ''Photorhabdus'' is known to be ...
'' bacterial symbionts of ''Heterorhabditis
''Heterorhabditis'' is a genus of nematodes belonging to the order Rhabditida. All species of this genus are obligate parasites of insects, and some are used as biological control agents for the control of pest insects.
''Heterorhabditis'' nemato ...
'' nematodes, producing 3,5-dihydroxy-4-isopropyl-trans-stilbene
Tapinarof, also known as benvitimod and sold under the brand name Vtama, is a medication used for the treatment of plaque psoriasis. The medication is applied to the skin. Besides its use in medicine, tapinarof is a naturally occurring compound ...
for antibiotic purposes.
Coumarin
Coumarin () or 2''H''-chromen-2-one is an aromatic organic chemical compound with formula . Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by a lactone-like chain , forming a second six-membered h ...
s biosynthesis
* Scopoletin glucosyltransferase
In enzymology, a scopoletin glucosyltransferase () is an enzyme that catalysis, catalyzes the chemical reaction
:UDP-glucose + scopoletin \rightleftharpoons UDP + scopolin
Thus, the two substrate (biochemistry), substrates of this enzyme are UDP- ...
, the enzyme that transforms scopoletin
Scopoletin is a coumarin. It found in the root of plants in the genus ''Scopolia'' such as ''Scopolia carniolica'' and ''Scopolia japonica'', in chicory, in '' Artemisia scoparia'', in the roots and leaves of stinging nettle (''Urtica dioica''), i ...
into scopolin
Scopolin is a glucoside of scopoletin formed by the action of the enzyme scopoletin glucosyltransferase.
References
Bibliography
*
*
O-methylated coumarins
Phenol glucosides
{{aromatic-stub ...
Chalcones biosynthesis
4-Coumaroyl-CoA
Coumaroyl-coenzyme A is the thioester of coenzyme-A and coumaric acid. Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include Monolignol, lignols (precursors to lignin ...
can be combined with malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid.
Functions
It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis.
Fatty acid biosynthesis
Malonyl-CoA provides 2-carbon units to fatty acids and commi ...
to yield the true backbone of flavonoids, a group of compounds called chalconoid
Chalconoids Greek: χαλκός ''khalkós'', "copper", due to its color), also known as ''chalcones'', are natural phenols related to chalcone. They form the central core for a variety of important biological compounds.
They show antibacterial, ...
s, which contain two phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
rings. Naringenin-chalcone synthase
Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase enzymes (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, a ...
is an enzyme that catalyzes the following conversion:
:3-malonyl-CoA + 4-coumaroyl-CoA → 4 CoA + naringenin chalcone
Naringenin chalcone is a common chalconoid (or chalcone, not to be confused with the compound chalcone). It is synthesized from 4-coumaroyl-CoA and malonyl-CoA by chalcone synthase (CHS), a key enzyme in the phenylpropanoid pathway. Naringenin ...
+ 3 CO2
Flavonoids biosynthesis
Conjugate ring-closure of chalcones results in the familiar form offlavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.
Chemically, flavonoids ...
s, the three-ringed structure of a flavone
Flavone is an organic compound with the formula . A white solid, flavone is a derivative of chromone with a phenyl (Ph) substituent adjacent to the ether group. The compound is of little direct practical importance, but susbstituted derivatives, t ...
.
Biodegradation
Hydroxycinnamic acids degradation
*Caffeate 3,4-dioxygenase
Caffeate 3,4-dioxygenase () is an enzyme that catalysis, catalyzes the chemical reaction
:3,4-dihydroxy-''trans''-cinnamate + O2 \rightleftharpoons 3-(2-carboxyethenyl)-''cis'',''cis''-muconate
Thus, the two substrate (biochemistry), substrates ...
is an enzyme that uses 3,4-dihydroxy-trans-cinnamate (caffeic acid
Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one ...
) and oxygen to produce 3-(2-carboxyethenyl)-cis,cis-muconate.
References
{{reflist Biosynthesis