|
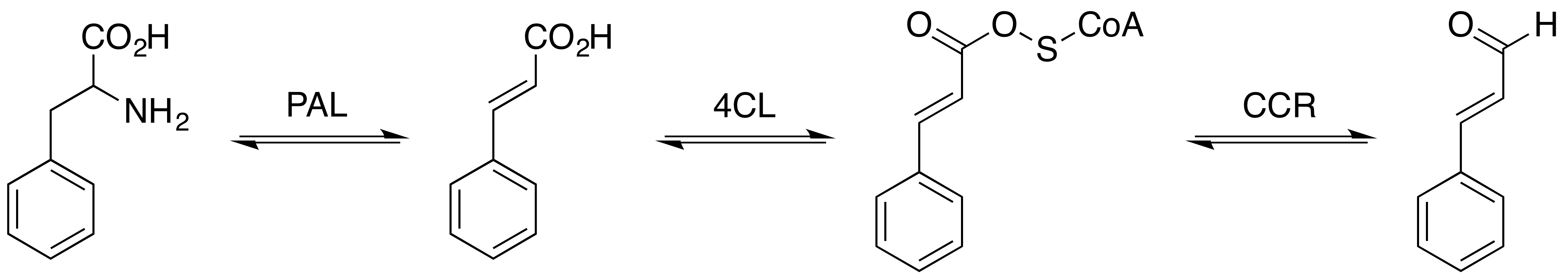
Cinnamoyl-CoA
Cinnamoyl-Coenzyme A is an intermediate in the phenylpropanoids metabolic pathway. Enzymes using Cinnamoyl-Coenzyme A * Cinnamoyl-CoA reductase, an enzyme that catalyzes the chemical reaction cinnamaldehyde + CoA + NADP+ → cinnamoyl-CoA + NADPH + H+ * Pinosylvin synthase, an enzyme that catalyzes the chemical reaction 3 malonyl-CoA + cinnamoyl-CoA → 4 CoA + pinosylvin Pinosylvin is an organic compound with the formula C6H5CH=CHC6H3(OH)2. A white solid, it is related to trans-stilbene, but with two hydroxy groups on one of the phenyl substituents. It is very soluble in many organic solvents, such as acetone. O ... + 4 CO2 * Cinnamoyl-CoA:phenyllactate CoA-transferase, an enzyme that catalyzes the chemical reaction (E)-cinnamoyl-CoA + (R)-phenyllactate → (E)-cinnamate + (R)-phenyllactyl-CoA References Thioesters of coenzyme A Cinnamate esters {{aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamoyl-CoA Reductase
Cinnamoyl-CoA reductase (), systematically named cinnamaldehyde:NADP+ oxidoreductase (CoA-cinnamoylating) but commonly referred to by the acronym CCR, is an enzyme that catalyzes the reduction of a substituted cinnamoyl-CoA to its corresponding cinnamaldehyde, utilizing NADPH and H+ and releasing free CoA and NADP+ in the process. Common biologically relevant cinnamoyl-CoA substrates for CCR include ''p''-coumaroyl-CoA and feruloyl-CoA, which are converted into ''p''-coumaraldehyde and coniferaldehyde, respectively, though most CCRs show activity toward a variety of other substituted cinnamoyl-CoA's as well. Catalyzing the first committed step in monolignol biosynthesis, this enzyme plays a critical role in lignin formation, a process important in plants both for structural development and defense response. Structure The first confirmed CCR was isolated from soybean (''Glycine max'') in 1976. However, crystal structures have so far been reported for just three CCR homologs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula(C9H8O) C6H5CH=CHCHO. Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus '' Cinnamomum''. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is ''trans''-cinnamaldehyde. The molecule consists of a benzene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinosylvin Synthase
In enzymology, a pinosylvin synthase () is an enzyme that catalyzes the chemical reaction :3 malonyl-CoA + cinnamoyl-CoA \rightleftharpoons 4 CoA + pinosylvin + 4 CO2 Thus, the two substrates of this enzyme are malonyl-CoA and cinnamoyl-CoA, whereas its 3 products are CoA, pinosylvin, and CO2. This enzyme belongs to the family of transferases, specifically those acyltransferases transferring groups other than aminoacyl groups. The systematic name of this enzyme class is malonyl-CoA:cinnamoyl-CoA malonyltransferase (cyclizing). Other names in common use include stilbene synthase, and pine stilbene synthase. This enzyme participates in phenylpropanoid biosynthesis The biosynthesis of phenylpropanoids involves a number of enzymes. From amino acids to cinnamates In plants, all phenylpropanoids are derived from the amino acids phenylalanine and tyrosine. Phenylalanine ammonia-lyase (PAL, a.k.a. phenylalanine/t .... References * EC 2.3.1 Enzymes of unknown structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid. Functions It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis. Fatty acid biosynthesis Malonyl-CoA provides 2-carbon units to fatty acids and commits them to fatty acid chain synthesis. Malonyl-CoA is formed by carboxylating acetyl-CoA using the enzyme acetyl-CoA carboxylase. One molecule of acetyl-CoA joins with a molecule of bicarbonate,Nelson D, Cox M (2008) ''Lehninger principles of biochemistry''. 5th Ed: p. 806 requiring energy rendered from ATP. Malonyl-CoA is utilised in fatty acid biosynthesis by the enzyme malonyl coenzyme A:acyl carrier protein transacylase (MCAT). MCAT serves to transfer malonate from malonyl-CoA to the terminal thiol of ''holo''- acyl carrier protein (ACP). Polyketide biosynthesis MCAT is also involved in bacterial polyketide biosynthesis. The enzyme MCAT together with an acyl carrier protein (ACP), and a polyketide synthase (PKS) and chain-len ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinosylvin
Pinosylvin is an organic compound with the formula C6H5CH=CHC6H3(OH)2. A white solid, it is related to trans-stilbene, but with two hydroxy groups on one of the phenyl substituents. It is very soluble in many organic solvents, such as acetone. Occurrence Pinosylvin is produced in plants in response to fungal infections, ozone-induced stress, and physical damage for example. It is a fungitoxin protecting the wood from fungal infection. It is present in the heartwood of ''Pinaceae'' and also found in '' Gnetum cleistostachyum''. Injected in rats, pinosylvin undergoes rapid glucuronidation and a poor bioavailability. Biosynthesis Pinosylvin synthase, an enzyme, catalyzes the biosynthesis of pinosylvin from malonyl-CoA and cinnamoyl-CoA: :3 malonyl-S-CoA + cinnamoyl-S-CoA → 4 CoA-SH + pinosylvin + 4 CO2 This biosynthesis is noteworthy because plant biosyntheses employing cinnamic acid as a starting point are rare compared to the more common use of ''p''-coumaric acid. Tw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
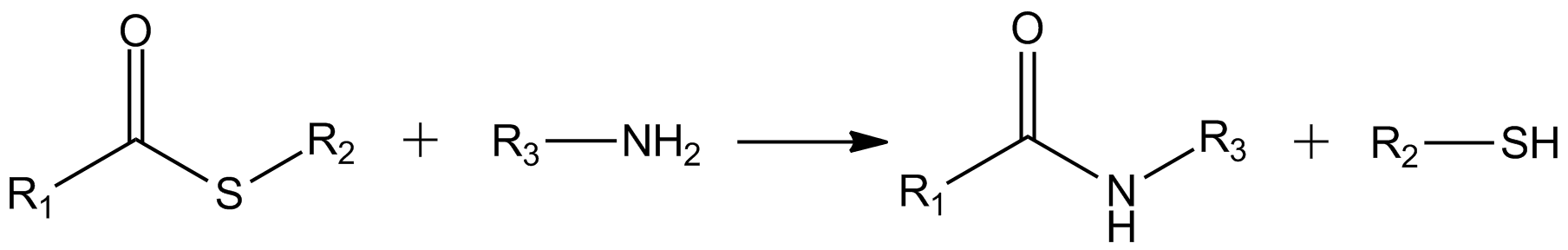
Thioesters Of Coenzyme A
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by the ''thio-'' prefix. They are the product of esterification between a carboxylic acid () and a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. Synthesis The most typical route to thioester involves the reaction of an acid chloride with an alkali metal salt of a thiol: :RSNa + R'COCl -> R'COSR + NaCl Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: :CH3C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |