persistent radical on:
[Wikipedia]
[Google]
[Amazon]

 In
In
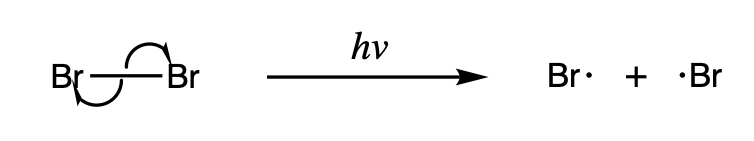
 Homolysis makes two new radicals from a spin-paired molecule by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond. Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light. The
Homolysis makes two new radicals from a spin-paired molecule by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond. Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light. The 



 Although organic radicals are generally stable intrinsically (in isolation), practically speaking their existence is only transient because they tend to dimerize. Some are quite long-lived. Generally organic radicals are stabilized by any or all of these factors: presence of electronegativity, delocalization, and steric hindrance. The compound 2,2,6,6-tetramethylpiperidinyloxyl illustrates the combination of all three factors. It is a commercially available solid that, aside from being magnetic, behaves like a normal organic compound.
Although organic radicals are generally stable intrinsically (in isolation), practically speaking their existence is only transient because they tend to dimerize. Some are quite long-lived. Generally organic radicals are stabilized by any or all of these factors: presence of electronegativity, delocalization, and steric hindrance. The compound 2,2,6,6-tetramethylpiperidinyloxyl illustrates the combination of all three factors. It is a commercially available solid that, aside from being magnetic, behaves like a normal organic compound.
 Delocalization effects can also be understood using
Delocalization effects can also be understood using  With a group that is instead electron-withdrawing, the SOMO then interacts with the empty π* orbital. There are no electrons occupying the higher energy orbital formed, while a new SOMO forms that is lower in energy. This results in a lower energy and higher stability of the radical species. Both donating groups and withdrawing groups stabilize radicals.
With a group that is instead electron-withdrawing, the SOMO then interacts with the empty π* orbital. There are no electrons occupying the higher energy orbital formed, while a new SOMO forms that is lower in energy. This results in a lower energy and higher stability of the radical species. Both donating groups and withdrawing groups stabilize radicals.
 Another well-known albeit weaker form of delocalization is
Another well-known albeit weaker form of delocalization is
 Most simply, the greater the steric hindrance the more difficult it is for reactions to take place, and the radical form is favored by default. For example, compare the hydrogen-abstracted form of ''N''-hydroxypiperidine to the molecule
Most simply, the greater the steric hindrance the more difficult it is for reactions to take place, and the radical form is favored by default. For example, compare the hydrogen-abstracted form of ''N''-hydroxypiperidine to the molecule
 Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as
Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as
 Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a
Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a 28th International Symposium on Free Radicals
.
 The homolytic cleavage of the breaking bond is drawn with a 'fish-hook' arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow. The second electron of the breaking bond also moves to pair up with the attacking radical electron; this is not explicitly indicated in this case.
Radicals also take part in
The homolytic cleavage of the breaking bond is drawn with a 'fish-hook' arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow. The second electron of the breaking bond also moves to pair up with the attacking radical electron; this is not explicitly indicated in this case.
Radicals also take part in

 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, a radical, also known as a free radical, is an atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
, molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
, or ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic ch ...
. Most organic radicals have short lifetimes.
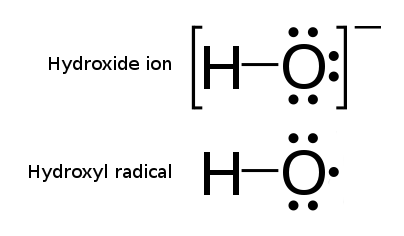
A notable example of a radical is the hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
(HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen
Triplet oxygen, 3O2, refers to the ''S'' = 1 electronic ground state of molecular oxygen (dioxygen). It is the most stable and common allotrope of oxygen. Molecules of triplet oxygen contain two unpaired electrons, making triplet oxygen an unus ...
and triplet carbene (꞉) which have two unpaired electrons.
Radicals may be generated in a number of ways, but typical methods involve redox reaction
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
s. Ionizing radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
, heat, electrical discharges, and electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations.
Radicals are important in combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
, atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary approach of research and draws on environmental chemistry, physics, meteoro ...
, polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are ...
, plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
chemistry, biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, and many other chemical processes. A majority of natural products are generated by radical-generating enzymes. In living organisms, the radicals superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the ...
and nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
and their reaction products regulate many processes, such as control of vascular tone and thus blood pressure. They also play a key role in the intermediary metabolism of various biological compounds. Such radicals can even be messengers in a process dubbed redox signaling
''Antioxidants & Redox Signaling '' is a peer-reviewed scientific journal covering reduction–oxidation (redox) signaling and antioxidant research. It covers topics such as reactive oxygen species/reactive nitrogen species (ROS/RNS) as messenge ...
. A radical may be trapped within a solvent cage
In chemistry, the cage effect (also known as geminate recombination) describes how the properties of a molecule are affected by its surroundings. First introduced by Franck and Rabinowitch in 1934, the cage effect suggests that instead of acting ...
or be otherwise bound.
Formation
Radicals are either (1) formed from spin-paired molecules or (2) from other radicals. Radicals are formed from spin-paired molecules through homolysis of weak bonds or electron transfer, also known as reduction. Radicals are formed from other radicals through substitution, addition, and elimination reactions.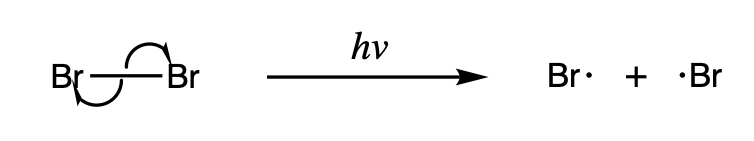
Radical formation from spin-paired molecules
Homolysis
 Homolysis makes two new radicals from a spin-paired molecule by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond. Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light. The
Homolysis makes two new radicals from a spin-paired molecule by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond. Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light. The bond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
associated with homolysis depends on the stability of a given compound, and some weak bonds are able to homolyze at relatively lower temperatures.
Some homolysis reactions are particularly important because they serve as an initiator for other radical reactions. One such example is the homolysis of halogens, which occurs under light and serves as the driving force for radical halogenation reactions.
Another notable reaction is the homolysis of dibenzoyl peroxide, which results in the formation of two benzoyloxy radicals and acts as an initiator for many radical reactions.

Reduction
Radicals can also form when a single electron is added to a spin-paired molecule, resulting in an electron transfer. This reaction, also called reduction, usually takes place with an alkali metal donating an electron to another spin-paired molecule.Radical formation from other radicals
Abstraction


Hydrogen abstraction
In chemistry, a hydrogen atom abstraction or hydrogen atom transfer (HAT) is any chemical reaction in which a hydrogen free radical (neutral hydrogen atom) is abstracted from a substrate according to the general equation:
:X^\bullet + H-Y -> X- ...
describes when a hydrogen atom is removed from a hydrogen donor molecule (e.g. tin or silicon hydride) with its one electron. Abstraction produces a new radical and a new spin-paired molecule. This is different from homolysis, which results in two radicals from a single spin-paired molecule and doesn’t include a radical as its reactant. Hydrogen abstraction is a fundamental process in radical chemistry because it serves as the final propagation step in many chemical reactions, converting carbon radicals into stable molecules. The figure to the right shows a radical abstraction between a benzoyloxy radical and a hydrogen bromide molecule, resulting in the production of a benzoic acid molecule and a bromine radical.
Addition
Radical addition
In organic chemistry, free-radical addition is an addition reaction which involves free radicals. The addition may occur between a radical and a non-radical, or between two radicals.
The basic steps with examples of the free-radical addition (al ...
describes when a radical is added to a spin-paired molecule to form a new radical. The figure on the right shows the addition of a bromine radical to an alkene. Radical addition follows the Anti -Markovnikov rule, where the substituent is added to the less substituted carbon atom.
Elimination
Radical elimination can be viewed as the reverse of radical addition. In radical elimination, an unstable radical compound breaks down into a spin-paired molecule and a new radical compound. Shown below is an example of a radical elimination reaction, where a benzoyloxy radical breaks down into a phenyl radical and a carbon dioxide molecule.
Stability
Stability of organic radicals
Electronegativity
Organic radicals are inherently electron deficient thus the greater the electronegativity of the atom on which the unpaired electron resides the less stable the radical. Between carbon, nitrogen, and oxygen, for example, carbon is the most stable and oxygen the least stable. Electronegativity also factors into the stability of carbon atoms of different hybridizations. Greater s-character correlates to higher electronegativity of the carbon atom (due to the close proximity of s orbitals to the nucleus), and the greater the electronegativity the less stable a radical. sp-hybridized carbons (50% s-character) form the least stable radicals compared to sp3-hybridized carbons (25% s-character) which form the most stable radicals.Delocalization
The delocalization of electrons across the structure of a radical, also known as its ability to form one or more resonance structures, allows for the electron-deficiency to be spread over several atoms, minimizing instability. Delocalization usually occurs in the presence of electron-donating groups, such as hydroxyl groups (−OH), ethers (−OR), adjacent alkenes, and amines (−NH2 or −NR), or electron-withdrawing groups, such as C=O or C≡N.molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecule ...
as a lens, more specifically, by examining the intramolecular interaction of the unpaired electron with a donating group’s pair of electrons or the empty π* orbital of an electron-withdrawing group in the form of a molecular orbital diagram. The HOMO of a radical is singly-occupied hence the orbital is aptly referred to as the SOMO, or the Singly-Occupied Molecular Orbital. For an electron-donating group, the SOMO interacts with the lower energy lone pair to form a new lower-energy filled bonding-orbital and a singly-filled new SOMO, higher in energy than the original. While the energy of the unpaired electron has increased, the decrease in energy of the lone pair forming the new bonding orbital outweighs the increase in energy of the new SOMO, resulting in a net decrease of the energy of the molecule. Therefore, electron-donating groups help stabilize radicals.
hyperconjugation
In organic chemistry, hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electron ...
. In radical chemistry, radicals are stabilized by hyperconjugation with adjacent alkyl groups. The donation of sigma (σ) C−H bonds into the partially empty radical orbitals helps to differentiate the stabilities of radicals on tertiary, secondary, and primary carbons. Tertiary carbon radicals have three σ C-H bonds that donate, secondary radicals only two, and primary radicals only one. Therefore, tertiary radicals are the most stable and primary radicals the least stable.
Steric hindrance
TEMPO
In musical terminology, tempo (Italian, 'time'; plural ''tempos'', or ''tempi'' from the Italian plural) is the speed or pace of a given piece. In classical music, tempo is typically indicated with an instruction at the start of a piece (often ...
. TEMPO, or (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl, is too sterically hindered by the additional methyl groups to react making it stable enough to be sold commercially in its radical form. ''N''-Hydroxypiperidine, however, does not have the four methyl groups to impede the way of a reacting molecule so the structure is unstable.
Facile H-atom donors
The stability of many (or most) organic radicals is not indicated by their isolability but is manifested in their ability to function as donors of H•. This property reflects a weakened bond to hydrogen, usually O−H but sometimes N−H or C−H. This behavior is important because these H• donors serve as antioxidants in biology and in commerce. Illustrative isα-tocopherol
α-Tocopherol is a type of vitamin E. It has E number "E307". Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free ra ...
(vitamin E
Vitamin E is a group of eight fat soluble compounds that include four tocopherols and four tocotrienols. Vitamin E deficiency, which is rare and usually due to an underlying problem with digesting dietary fat rather than from a diet low in vitami ...
). The tocopherol radical itself is insufficiently stable for isolation, but the parent molecule is a highly effective hydrogen-atom donor. The C−H bond is weakened in triphenylmethyl
The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trit ...
(trityl) derivatives.
upright=1.1, 2,2,6,6-Tetramethylpiperidinyloxyl is an example of a robust organic radical.
Inorganic radicals
A large variety of inorganic radicals are stable and in fact isolable. Examples include most first-row transition metal complexes. With regard to main group radicals, the most abundant radical in the universe is also the most abundant chemical in the universe, H•. Most main group radicals are not however ''isolable'', despite their intrinsic stability. Hydrogen radicals for example combine eagerly to form H2.Nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
(NO) is well known example of an isolable inorganic radical. Fremy's salt (Potassium nitrosodisulfonate, (KSO3)2NO) is a related example. Many thiazyl radicals are known, despite limited extent of π resonance stabilization
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
.
Many radicals can be envisioned as the products of breaking of covalent bonds by homolysis. The homolytic bond dissociation energies
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
, usually abbreviated as "Δ''H''°" are a measure of bond strength. Splitting H2 into 2 H•, for example, requires a Δ''H''° of +435 kJ/mol, while splitting Cl2 into two Cl• requires a Δ''H''° of +243 kJ/mol. For weak bonds, homolysis can be induced thermally. Strong bonds require high energy photons or even flames to induce homolysis.
Diradicals
Diradical
In chemistry, a diradical is a molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactive and i ...
s are molecules containing two radical centers. Dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
*A ...
(O2) is an important example of a stable diradical. Singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
, the lowest-energy non-radical state of dioxygen, is less stable than the diradical due to Hund's rule of maximum multiplicity
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configurati ...
. The relative stability of the oxygen diradical is primarily due to the spin-forbidden nature of the triplet-singlet transition required for it to grab electrons, i.e., "oxidize". The diradical state of oxygen also results in its paramagnetic character, which is demonstrated by its attraction to an external magnet. Diradicals can also occur in metal-oxo complexes, lending themselves for studies of spin forbidden reactions in transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
chemistry. Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s in their triplet state can be viewed as diradicals centred on the same atom, while these are usually highly reactive persistent carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
s are known, with N-heterocyclic carbenes being the most common example.
Triplet carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s and nitrene
In chemistry, a nitrene or imene () is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and univalent, so it has only 6 electrons in its valence level—two covalent bonded and four non-bonded electrons. It is therefore consid ...
s are diradicals. Their chemical properties are distinct from the properties of their singlet analogues.
Occurrence of radicals
Combustion
A familiar radical reaction iscombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
. The oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
molecule is a stable diradical
In chemistry, a diradical is a molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactive and i ...
, best represented by •O–O•. Because spins
The spins (as in having "the spins")Diane Marie Leiva. ''The Florida State University College of Education''Women's Voices on College Drinking: The First-Year College Experience"/ref> is an adverse reaction of intoxication that causes a state of v ...
of the electrons are parallel, this molecule is stable. While the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
of oxygen is this unreactive spin-unpaired ( triplet) diradical, an extremely reactive spin-paired ( singlet) state is available. For combustion to occur, the energy barrier
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
between these must be overcome. This barrier can be overcome by heat, requiring high temperatures. The triplet-singlet transition is also " forbidden". This presents an additional barrier to the reaction. It also means molecular oxygen is relatively unreactive at room temperature except in the presence of a catalytic heavy atom such as iron or copper.
Combustion consists of various radical chain reactions that the singlet radical can initiate. The flammability
A combustible material is something that can burn (i.e., ''combust'') in air. A combustible material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort and a flammable mat ...
of a given material strongly depends on the concentration of radicals that must be obtained before initiation and propagation reactions dominate leading to combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
of the material. Once the combustible material has been consumed, termination reactions again dominate and the flame dies out. As indicated, promotion of propagation or termination reactions alters flammability. For example, because lead itself deactivates radicals in the gasoline-air mixture, tetraethyl lead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula Pb( C2H5)4. It is a fuel additive, first being mixed with gasoline beginning in the 1920s as a patented octane rating booster that al ...
was once commonly added to gasoline. This prevents the combustion from initiating in an uncontrolled manner or in unburnt residues (engine knocking
In spark ignition internal combustion engines, knocking (also knock, detonation, spark knock, pinging or pinking) occurs when combustion of some of the air/fuel mixture in the cylinder does not result from propagation of the flame front ignite ...
) or premature ignition (preignition
In spark ignition internal combustion engines, knocking (also knock, detonation, spark knock, pinging or pinking) occurs when combustion of some of the air/fuel mixture in the cylinder does not result from propagation of the flame front ignite ...
).
When a hydrocarbon is burned, a large number of different oxygen radicals are involved. Initially, hydroperoxyl radical (HOO•) are formed. These then react further to give organic hydroperoxides that break up into hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
s (HO•).
Polymerization
Manypolymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are ...
reactions are initiated by radicals. Polymerization involves an initial radical adding to non-radical (usually an alkene) to give new radicals. This process is the basis of the radical chain reaction. The art of polymerization entails the method by which the initiating radical is introduced. For example, methyl methacrylate
Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA ...
(MMA) can be polymerized to produce Poly(methyl methacrylate)
Poly(methyl methacrylate) (PMMA) belongs to a group of materials called engineering plastics. It is a transparent thermoplastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and brands Crylux, Plexiglas, Acrylite, ...
(PMMA - Plexiglas or Perspex) via a repeating series of radical addition
In organic chemistry, free-radical addition is an addition reaction which involves free radicals. The addition may occur between a radical and a non-radical, or between two radicals.
The basic steps with examples of the free-radical addition (al ...
steps:
upright=3.35, center, Radical intermediates in the formation of polymethacrylate (plexiglas or perspex)
Newer radical polymerization methods are known as living radical polymerization. Variants include reversible addition-fragmentation chain transfer (RAFT
A raft is any flat structure for support or transportation over water. It is usually of basic design, characterized by the absence of a hull. Rafts are usually kept afloat by using any combination of buoyant materials such as wood, sealed barrel ...
) and atom transfer radical polymerization ( ATRP).
Being a prevalent radical, O2 reacts with many organic compounds to generate radicals together with the hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. ...
radical. Drying oil
A drying oil is an oil that hardens to a tough, solid film after a period of exposure to air, at room temperature. The oil hardens through a chemical reaction in which the components crosslink (and hence, polymerize) by the action of oxygen (not ...
s and alkyd paints harden due to radical crosslinking initiated by oxygen from the atmosphere.
Atmospheric radicals
The most common radical in the lower atmosphere is molecular dioxygen.Photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
of source molecules produces other radicals. In the lower atmosphere, important radical are produced by the photodissociation of nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the producti ...
to an oxygen atom and nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
(see below), which plays a key role in smog
Smog, or smoke fog, is a type of intense air pollution. The word "smog" was coined in the early 20th century, and is a portmanteau of the words ''smoke'' and '' fog'' to refer to smoky fog due to its opacity, and odor. The word was then inte ...
formation—and the photodissociation of ozone to give the excited oxygen atom O(1D) (see below). The net and return reactions are also shown ( and , respectively).
In the upper atmosphere, the photodissociation of normally unreactive chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and prop ...
s (CFCs) by solar ultraviolet radiation
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
is an important source of radicals (see eq. 1 below). These reactions give the chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
radical, Cl•, which catalyzes the conversion of ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
to O2, thus facilitating ozone depletion
Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone l ...
(– below).
Such reactions cause the depletion of the ozone layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in rela ...
, especially since the chlorine radical is free to engage in another reaction chain; consequently, the use of chlorofluorocarbons as refrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Ref ...
s has been restricted.
In biology
 Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as
Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as granulocyte
Granulocytes are
cells in the innate immune system characterized by the presence of specific granules in their cytoplasm. Such granules distinguish them from the various agranulocytes. All myeloblastic granulocytes are polymorphonuclear. They ha ...
s and macrophage
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer cel ...
s. Radicals are involved in cell signalling
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellula ...
processes, known as redox signaling
''Antioxidants & Redox Signaling '' is a peer-reviewed scientific journal covering reduction–oxidation (redox) signaling and antioxidant research. It covers topics such as reactive oxygen species/reactive nitrogen species (ROS/RNS) as messenge ...
. For example, radical attack of linoleic acid produces a series of 13-hydroxyoctadecadienoic acids and 9-hydroxyoctadecadienoic acid
9-Hydroxyoctadecadienoic acid (or 9-HODE) has been used in the literature to designate either or both of two stereoisomer metabolites of the essential fatty acid, linoleic acid: 9(''S'')-hydroxy-10(''E''),12(''Z'')-octadecadienoic acid (9(''S'')- ...
s, which may act to regulate localized tissue inflammatory and/or healing responses, pain perception, and the proliferation of malignant cells. Radical attacks on arachidonic acid and docosahexaenoic acid produce a similar but broader array of signaling products.
Radicals may also be involved in Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. The symptoms usually emerge slowly, and as the disease worsens, non-motor symptoms becom ...
, senile and drug-induced deafness
Deafness has varying definitions in cultural and medical contexts. In medical contexts, the meaning of deafness is hearing loss that precludes a person from understanding spoken language, an audiological condition. In this context it is written ...
, schizophrenia
Schizophrenia is a mental disorder characterized by continuous or relapsing episodes of psychosis. Major symptoms include hallucinations (typically hearing voices), delusions, and disorganized thinking. Other symptoms include social withdra ...
, and Alzheimer's
Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens. It is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As t ...
. The classic free-radical syndrome, the iron-storage disease hemochromatosis
Iron overload or hemochromatosis (also spelled ''haemochromatosis'' in British English) indicates increased total accumulation of iron in the body from any cause and resulting organ damage. The most important causes are hereditary haemochromatosi ...
, is typically associated with a constellation of free-radical-related symptoms including movement disorder, psychosis, skin pigmentary melanin
Melanin (; from el, μέλας, melas, black, dark) is a broad term for a group of natural pigments found in most organisms. Eumelanin is produced through a multistage chemical process known as melanogenesis, where the oxidation of the amino ...
abnormalities, deafness, arthritis, and diabetes mellitus. The free-radical theory
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spo ...
of aging proposes that radicals underlie the aging process
Ageing ( BE) or aging ( AE) is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In ...
itself. Similarly, the process of mitohormesis
Hormesis is a characteristic of many biological processes, namely a biphasic or triphasic response to exposure to increasing amounts of a substance or condition. Within the hormetic zone, the biological response to low exposures to toxins and othe ...
suggests that repeated exposure to radicals may extend life span.
Because radicals are necessary for life, the body has a number of mechanisms to minimize radical-induced damage and to repair damage that occurs, such as the enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s superoxide dismutase
Superoxide dismutase (SOD, ) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide () radical into ordinary molecular oxygen (O2) and hydrogen peroxide (). Superoxide is produced as a by-product of oxygen me ...
, catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting t ...
, glutathione peroxidase
Glutathione peroxidase (GPx) () is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid h ...
and glutathione reductase
Glutathione reductase (GR) also known as glutathione-disulfide reductase (GSR) is an enzyme that in humans is encoded by the GSR gene. Glutathione reductase (EC 1.8.1.7) catalyzes the reduction of glutathione disulfide (GSSG) to the sulfhydryl fo ...
. In addition, antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricant ...
s play a key role in these defense mechanisms. These are often the three vitamins, vitamin A
Vitamin A is a fat-soluble vitamin and an essential nutrient for humans. It is a group of organic compounds that includes retinol, retinal (also known as retinaldehyde), retinoic acid, and several provitamin A carotenoids (most notably bet ...
, vitamin C
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) an ...
and vitamin E
Vitamin E is a group of eight fat soluble compounds that include four tocopherols and four tocotrienols. Vitamin E deficiency, which is rare and usually due to an underlying problem with digesting dietary fat rather than from a diet low in vitami ...
and polyphenol antioxidants. Furthermore, there is good evidence indicating that bilirubin
Bilirubin (BR) (Latin for "red bile") is a red-orange compound that occurs in the normal catabolic pathway that breaks down heme in vertebrates. This catabolism is a necessary process in the body's clearance of waste products that arise from the ...
and uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of ...
can act as antioxidants to help neutralize certain radicals. Bilirubin comes from the breakdown of red blood cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
s' contents, while uric acid is a breakdown product of purine
Purine is a heterocyclic compound, heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which includ ...
s. Too much bilirubin, though, can lead to jaundice
Jaundice, also known as icterus, is a yellowish or greenish pigmentation of the skin and sclera due to high bilirubin levels. Jaundice in adults is typically a sign indicating the presence of underlying diseases involving abnormal heme meta ...
, which could eventually damage the central nervous system, while too much uric acid causes gout
Gout ( ) is a form of inflammatory arthritis characterized by recurrent attacks of a red, tender, hot and swollen joint, caused by deposition of monosodium urate monohydrate crystals. Pain typically comes on rapidly, reaching maximal intensit ...
.
Reactive oxygen species
Reactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
or ROS are species such as superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the ...
, hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
, and hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
, commonly associated with cell damage. ROS form as a natural by-product of the normal metabolism of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
and have important roles in cell signaling.
Two important oxygen-centered radicals are superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the ...
and hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
. They derive from molecular oxygen under reducing conditions. However, because of their reactivity, these same radicals can participate in unwanted side reactions resulting in cell damage. Excessive amounts of these radicals can lead to cell injury and death
Death is the irreversible cessation of all biological functions that sustain an organism. For organisms with a brain, death can also be defined as the irreversible cessation of functioning of the whole brain, including brainstem, and brain ...
, which may contribute to many diseases such as cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
, stroke
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functionin ...
, myocardial infarction
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may ...
, diabetes
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level ( hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ap ...
and major disorders. Many forms of cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
are thought to be the result of reactions between radicals and DNA, potentially resulting in mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mi ...
s that can adversely affect the cell cycle
The cell cycle, or cell-division cycle, is the series of events that take place in a cell that cause it to divide into two daughter cells. These events include the duplication of its DNA (DNA replication) and some of its organelles, and subs ...
and potentially lead to malignancy. Some of the symptoms of aging
Ageing ( BE) or aging ( AE) is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In ...
such as atherosclerosis
Atherosclerosis is a pattern of the disease arteriosclerosis in which the wall of the artery develops abnormalities, called lesions. These lesions may lead to narrowing due to the buildup of atheroma, atheromatous plaque. At onset there are usu ...
are also attributed to radical induced oxidation of cholesterol to 7-ketocholesterol. In addition radicals contribute to alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
-induced liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
damage, perhaps more than alcohol itself. Radicals produced by cigarette
A cigarette is a narrow cylinder containing a combustible material, typically tobacco, that is rolled into thin paper for smoking. The cigarette is ignited at one end, causing it to smolder; the resulting smoke is orally inhaled via the opp ...
smoke
Smoke is a suspension of airborne particulates and gases emitted when a material undergoes combustion or pyrolysis, together with the quantity of air that is entrained or otherwise mixed into the mass. It is commonly an unwanted by-product ...
are implicated in inactivation of alpha 1-antitrypsin
Alpha-1 antitrypsin or α1-antitrypsin (A1AT, α1AT, A1A, or AAT) is a protein belonging to the serpin superfamily. It is encoded in humans by the ''SERPINA1'' gene. A protease inhibitor, it is also known as alpha1–proteinase inhibitor (A1PI) ...
in the lung
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of t ...
. This process promotes the development of emphysema
Emphysema, or pulmonary emphysema, is a lower respiratory tract disease, characterised by air-filled spaces ( pneumatoses) in the lungs, that can vary in size and may be very large. The spaces are caused by the breakdown of the walls of the alve ...
.
Oxybenzone
Oxybenzone or benzophenone-3 or BP-3 (trade names Milestab 9, Eusolex 4360, Escalol 567, KAHSCREEN BZ-3) is an organic compound. It is a pale-yellow solid that is readily soluble in most organic solvents. Oxybenzone belongs to the class of aroma ...
has been found to form radicals in sunlight, and therefore may be associated with cell damage as well. This only occurred when it was combined with other ingredients commonly found in sunscreens, like titanium oxide
Titanium oxide may refer to:
* Titanium dioxide (titanium(IV) oxide), TiO2
* Titanium(II) oxide (titanium monoxide), TiO, a non-stoichiometric oxide
* Titanium(III) oxide (dititanium trioxide), Ti2O3
* Ti3O
* Ti2O
* δ-TiOx (x= 0.68–0.75)
* T ...
and octyl methoxycinnamate
Octyl methoxycinnamate or ethylhexyl methoxycinnamate (INCI) or octinoxate (USAN), trade names Eusolex 2292 and Uvinul MC80, is an organic compound that is an ingredient in some sunscreens and lip balms. It is an ester formed from methoxycinnam ...
.
ROS attack the polyunsaturated fatty acid
Polyunsaturated fatty acids (PUFAs) are fatty acids that contain more than one double bond in their backbone. This class includes many important compounds, such as essential fatty acids and those that give drying oils their characteristic proper ...
, linoleic acid
Linoleic acid (LA) is an organic compound with the formula COOH(CH2)7CH=CHCH2CH=CH(CH2)4CH3. Both alkene groups are cis-trans isomerism, ''cis''. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 ''cis''-9,12. A linoleate is a salt (chem ...
, to form a series of 13-hydroxyoctadecadienoic acid and 9-hydroxyoctadecadienoic acid
9-Hydroxyoctadecadienoic acid (or 9-HODE) has been used in the literature to designate either or both of two stereoisomer metabolites of the essential fatty acid, linoleic acid: 9(''S'')-hydroxy-10(''E''),12(''Z'')-octadecadienoic acid (9(''S'')- ...
products that serve as signaling molecules that may trigger responses that counter the tissue injury which caused their formation. ROS attacks other polyunsaturated fatty acids, e.g. arachidonic acid
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). It is structurally related to the saturated arachidic acid found in cupuaçu butter. Its name derives from the New Latin word ''arachi ...
and docosahexaenoic acid
Docosahexaenoic acid (DHA) is an omega-3 fatty acid that is a primary structural component of the human brain, cerebral cortex, skin, and retina. In physiological literature, it is given the name 22:6(n-3). It can be synthesized from alpha-lino ...
, to produce a similar series of signaling products.
History and nomenclature
 Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a
Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
or a carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
, whether it was part of a larger molecule or a molecule on its own. The qualifier "free" was then needed to specify the unbound case. Following recent nomenclature revisions, a part of a larger molecule is now called a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
or substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
, and "radical" now implies "free". However, the old nomenclature may still appear in some books.
The term radical was already in use when the now obsolete radical theory Radical theory is an obsolete scientific theory in chemistry describing the structure of organic compounds. The theory was pioneered by Justus von Liebig, Friedrich Wöhler and Auguste Laurent around 1830 and is not related to the modern understand ...
was developed. Louis-Bernard Guyton de Morveau
Louis-Bernard Guyton, Baron de Morveau (also Louis-Bernard Guyton-Morveau after the French Revolution; 4 January 1737 – 2 January 1816) was a French chemist, politician, and aeronaut. He is credited with producing the first systematic method o ...
introduced the phrase "radical" in 1785 and the phrase was employed by Antoine Lavoisier
Antoine-Laurent de Lavoisier ( , ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and th ...
in 1789 in his Traité Élémentaire de Chimie
''Traité élémentaire de chimie'' (''Elementary Treatise on Chemistry'') is a textbook written by Antoine Lavoisier published in 1789 and translated into English by Robert Kerr in 1790 under the title ''Elements of Chemistry in a New Systemati ...
. A radical was then identified as the root base of certain acids (the Latin word "radix" meaning "root"). Historically, the term ''radical'' in radical theory Radical theory is an obsolete scientific theory in chemistry describing the structure of organic compounds. The theory was pioneered by Justus von Liebig, Friedrich Wöhler and Auguste Laurent around 1830 and is not related to the modern understand ...
was also used for bound parts of the molecule, especially when they remain unchanged in reactions. These are now called functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s. For example, methyl alcohol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
was described as consisting of a methyl "radical" and a hydroxyl "radical". Neither are radicals in the modern chemical sense, as they are permanently bound to each other, and have no unpaired, reactive electrons; however, they can be observed as radicals in mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
when broken apart by irradiation with energetic electrons.
In a modern context the first organic (carbon–containing) radical identified was the triphenylmethyl radical
The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the tri ...
, (C6H5)3C•. This species was discovered by Moses Gomberg
Moses Gomberg (February 8, 1866 – February 12, 1947) was a chemistry professor at the University of Michigan. He was elected to the National Academy of Sciences and served as president of the American Chemical Society.
Early life and education
...
in 1900. In 1933 Morris S. Kharasch and Frank Mayo proposed that free radicals were responsible for anti-Markovnikov addition
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a ...
of hydrogen bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temper ...
to allyl bromide
Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, synthetic perfumes and other organic compounds. Physically, allyl bromide is a colorless liquid with an irritating and p ...
.
In most fields of chemistry, the historical definition of radicals contends that the molecules have nonzero electron spin. However, in fields including spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
and astrochemistry
Astrochemistry is the study of the abundance and reactions of molecules in the Universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar Syst ...
, the definition is slightly different. Gerhard Herzberg
Gerhard Heinrich Friedrich Otto Julius Herzberg, (; December 25, 1904 – March 3, 1999) was a German-Canadian pioneering physicist and physical chemist, who won the Nobel Prize for Chemistry in 1971, "for his contributions to the knowledge o ...
, who won the Nobel prize for his research into the electron structure and geometry of radicals, suggested a looser definition of free radicals: "any transient (chemically unstable) species (atom, molecule, or ion)". The main point of his suggestion is that there are many chemically unstable molecules that have zero spin, such as C2, C3, CH2 and so on. This definition is more convenient for discussions of transient chemical processes and astrochemistry; therefore researchers in these fields prefer to use this loose definition..
Depiction in chemical reactions
In chemical equations, radicals are frequently denoted by a dot placed immediately to the right of the atomic symbol or molecular formula as follows: : Radicalreaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
s use single-headed arrows to depict the movement of single electrons:
radical addition
In organic chemistry, free-radical addition is an addition reaction which involves free radicals. The addition may occur between a radical and a non-radical, or between two radicals.
The basic steps with examples of the free-radical addition (al ...
and radical substitution
In organic chemistry, a radical-substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.March Jerry; (1985). Advanced organic chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wile ...
as reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s. Chain reactions involving radicals can usually be divided into three distinct processes. These are ''initiation'', ''propagation'', and ''termination''.
*''Initiation'' reactions are those that result in a net increase in the number of radicals. They may involve the formation of radicals from stable species as in Reaction 1 above or they may involve reactions of radicals with stable species to form more radicals.
*''Propagation'' reactions are those reactions involving radicals in which the total number of radicals remains the same.
*''Termination'' reactions are those reactions resulting in a net decrease in the number of radicals. Typically two radicals combine to form a more stable species, for example:
*:2 Cl• → Cl2
See also
*Electron pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper he ...
* Globally Harmonized System of Classification and Labelling of Chemicals
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) is an internationally agreed-upon standard managed by the United Nations that was set up to replace the assortment of hazardous material classification and labelli ...
* Hofmann–Löffler reaction
The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 ( pyrrolidine or, ...
;Free radical research
* ARC Centre of Excellence for Free Radical Chemistry and Biotechnology
References
{{Authority control Articles containing video clips Biological processes Biomolecules Chemical bonding Environmental chemistry Senescence