
Ozone depletion consists of two related events observed since the late 1970s: a lowered total amount of
ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
in
Earth's upper atmosphere, and a much larger springtime decrease in
stratospheric ozone (the
ozone layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorption (electromagnetic radiation), absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the a ...
) around Earth's polar regions.
The latter phenomenon is referred to as the
ozone hole
Ozone depletion consists of two related events observed since the late 1970s: a lowered total amount of ozone in Earth, Earth's upper atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone layer) around Earth's polar ...
. There are also springtime polar
tropospheric ozone depletion events in addition to these stratospheric events.
The main causes of ozone depletion and the ozone hole are manufactured chemicals, especially manufactured
halocarbon
Halocarbon compounds are chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, or ...
refrigerant
A refrigerant is a working fluid used in the cooling, heating, or reverse cooling/heating cycles of air conditioning systems and heat pumps, where they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are ...
s,
solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s,
propellant
A propellant (or propellent) is a mass that is expelled or expanded in such a way as to create a thrust or another motive force in accordance with Newton's third law of motion, and "propel" a vehicle, projectile, or fluid payload. In vehicle ...
s, and foam-
blowing agents (
chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly Halogenation, halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatility (chemistry), volat ...
s (CFCs), HCFCs,
halons), referred to as ''ozone-depleting substances'' (ODS). These compounds are transported into the
stratosphere
The stratosphere () is the second-lowest layer of the atmosphere of Earth, located above the troposphere and below the mesosphere. The stratosphere is composed of stratified temperature zones, with the warmer layers of air located higher ...
by
turbulent mixing after being emitted from the surface, mixing much faster than the molecules can settle. Once in the stratosphere, they release
atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
from the
halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
group through
photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
, which
catalyze the breakdown of ozone (O
3) into oxygen (O
2). Both types of ozone depletion were observed to increase as emissions of halocarbons increased.
Ozone depletion and the ozone hole have generated worldwide concern over increased cancer risks and other negative effects. The ozone layer prevents harmful wavelengths of
ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
(UVB) light from passing through the
Earth's atmosphere
The atmosphere of Earth is composed of a layer of gas mixture that surrounds the Earth's planetary surface (both lands and oceans), known collectively as air, with variable quantities of suspended aerosols and particulates (which create weathe ...
. These wavelengths cause
skin cancer
Skin cancers are cancers that arise from the Human skin, skin. They are due to the development of abnormal cells (biology), cells that have the ability to invade or metastasis, spread to other parts of the body. It occurs when skin cells grow ...
,
sunburn
Sunburn is a form of radiation burn that affects living tissue, such as skin, that results from an overexposure to ultraviolet (UV) radiation, usually from the Sun. Common symptoms in humans and other animals include red or reddish skin tha ...
, permanent blindness, and
cataracts
A cataract is a cloudy area in the lens of the eye that leads to a decrease in vision of the eye. Cataracts often develop slowly and can affect one or both eyes. Symptoms may include faded colours, blurry or double vision, halos around ligh ...
, which were projected to increase dramatically as a result of thinning ozone, as well as harming plants and animals. These concerns led to the adoption of the
Montreal Protocol
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 ...
in 1987, which bans the production of CFCs, halons, and other ozone-depleting chemicals. Over time, scientists have developed new refrigerants with lower
global warming potential
Global warming potential (GWP) is a measure of how much heat a greenhouse gas traps in the atmosphere over a specific time period, relative to carbon dioxide (). It is expressed as a multiple of warming caused by the same mass of carbon dioxide ( ...
(GWP) to replace older ones. For example, in new automobiles,
R-1234yf systems are now common, being chosen over refrigerants with much higher GWP such as
R-134a and
R-12.
The ban came into effect in 1989. Ozone levels stabilized by the mid-1990s and began to recover in the 2000s, as the shifting of the
jet stream
Jet streams are fast flowing, narrow thermal wind, air currents in the Earth's Atmosphere of Earth, atmosphere.
The main jet streams are located near the altitude of the tropopause and are westerly winds, flowing west to east around the gl ...
in the southern hemisphere towards the south pole has stopped and might even be reversing. Recovery was projected to continue over the next century, with the ozone hole expected to reach pre-1980 levels by around 2075.
In 2019,
NASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the federal government of the United States, US federal government responsible for the United States ...
reported that the ozone hole was the smallest ever since it was first discovered in 1982.
The UN now projects that under the current regulations the ozone layer will completely regenerate by 2045. The Montreal Protocol is considered the most successful international environmental agreement to date.
Ozone cycle overview

Three forms (or
allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
) of
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
are involved in the
ozone-oxygen cycle: oxygen atoms (O or atomic oxygen), oxygen gas ( or diatomic oxygen), and ozone gas ( or triatomic oxygen). Ozone is formed in the stratosphere when oxygen gas molecules photodissociate after absorbing UVC photons. This converts a single into two atomic oxygen
radicals. The atomic oxygen radicals then combine with separate molecules to create two molecules. These ozone molecules absorb UVB light, following which ozone splits into a molecule of and an oxygen atom. The oxygen atom then joins up with an oxygen molecule to regenerate ozone. This is a continuing process that terminates when an oxygen atom recombines with an ozone molecule to make two molecules. It is worth noting that ozone is the only atmospheric gas that absorbs UVB light.
:O + → 2


The total amount of ozone in the stratosphere is determined by a balance between photochemical production and recombination.
Ozone can be destroyed by a number of
free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
catalysts; the most important are the
hydroxyl radical
The hydroxyl radical, •HO, is the neutral form of the hydroxide ion (HO–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are pr ...
(OH·),
nitric oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes den ...
radical (NO·),
chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
radical (Cl·) and
bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
radical (Br·). The dot is a notation to indicate that each species has an unpaired electron and is thus extremely reactive. The effectiveness of different
halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s and
pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
s as catalysts for ozone destruction varies, in part due to differing routes to regenerate the original radical after reacting with ozone or dioxygen.
While all of the relevant radicals have both natural and man-made sources, human activity has impacted some more than others. As of 2020, most of the OH· and NO· in the stratosphere is naturally occurring, but human activity has drastically increased the levels of chlorine and bromine. These elements are found in stable organic compounds, especially
chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly Halogenation, halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatility (chemistry), volat ...
s, which can travel to the stratosphere without being destroyed in the troposphere due to their low reactivity. Once in the stratosphere, the Cl and Br atoms are released from the parent compounds by the action of ultraviolet light, e.g.
: +
electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
→ Cl· + ·
Ozone is a highly reactive molecule that easily reduces to the more stable oxygen form with the assistance of a catalyst. Cl and Br atoms destroy ozone molecules through a variety of
catalytic
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
cycles. In the simplest example of such a cycle, a chlorine atom reacts with an ozone molecule (), taking an oxygen atom to form chlorine monoxide (ClO) and leaving an oxygen molecule (). The ClO can react with a second molecule of ozone, releasing the chlorine atom and yielding two molecules of oxygen. The chemical shorthand for these gas-phase reactions is:
* Cl· + → ClO +
A chlorine atom removes an oxygen atom from an ozone molecule to make a ClO molecule
* ClO + → Cl· + 2
This ClO can also remove an oxygen atom from another ozone molecule; the chlorine is free to repeat this two-step cycle
The overall effect is a decrease in the amount of ozone, though the rate of these processes can be decreased by the effects of
null cycles. More complicated mechanisms have also been discovered that lead to ozone destruction in the lower stratosphere.
A single chlorine atom would continuously destroy ozone (thus a catalyst) for up to two years (the time scale for transport back down to the troposphere) except for reactions that remove it from this cycle by forming reservoir species such as
hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
(HCl) and
chlorine nitrate (). Bromine is even more efficient than chlorine at destroying ozone on a per-atom basis, but there is much less bromine in the atmosphere at present. Both chlorine and bromine contribute significantly to overall ozone depletion. Laboratory studies have also shown that fluorine and iodine atoms participate in analogous catalytic cycles. However, fluorine atoms react rapidly with water vapour, methane and hydrogen to form strongly bound
hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
(HF) in the Earth's stratosphere, while organic molecules containing iodine react so rapidly in the lower atmosphere that they do not reach the stratosphere in significant quantities.
A single chlorine atom is able to react with an average of 100,000 ozone molecules before it is removed from the catalytic cycle. This fact plus the amount of chlorine released into the atmosphere yearly by chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) demonstrates the danger of CFCs and HCFCs to the environment.
Observations on ozone layer depletion
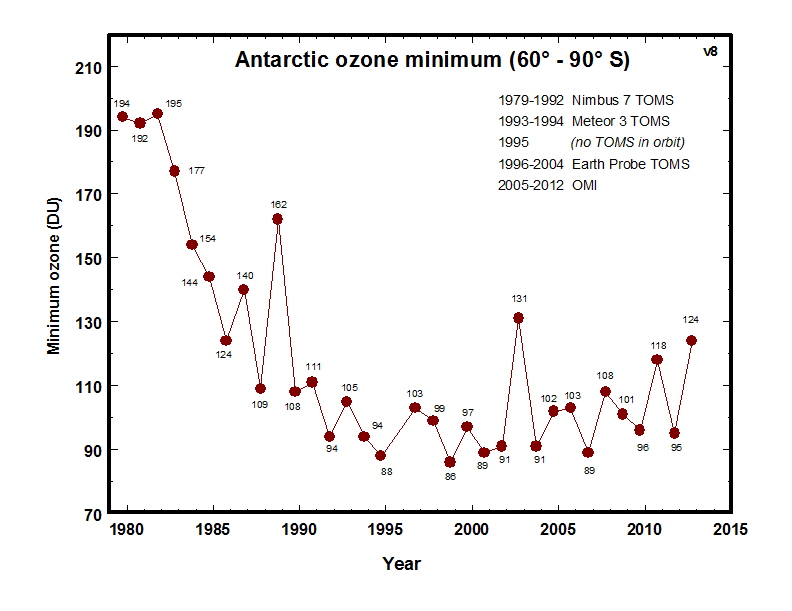
The ozone hole is usually measured by reduction in the total ''column ozone'' above a point on the Earth's surface. This is normally expressed in
Dobson units; abbreviated as "DU". The most prominent decrease in ozone has been in the lower stratosphere. Marked decreases in column ozone in the
Antarctic
The Antarctic (, ; commonly ) is the polar regions of Earth, polar region of Earth that surrounds the South Pole, lying within the Antarctic Circle. It is antipodes, diametrically opposite of the Arctic region around the North Pole.
The Antar ...
spring and early summer compared to the early 1970s and before have been observed using instruments such as the
Total Ozone Mapping Spectrometer
The Total Ozone Mapping Spectrometer (TOMS) was a NASA satellite instrument, specifically a spectrometer, for measuring the ozone layer. Of the five TOMS instruments which were built, four entered successful orbit. The satellites carrying TOMS ins ...
(TOMS).
Reductions of up to 70 percent in the ozone column observed in the austral (southern hemispheric) spring over Antarctica and first reported in 1985 (Farman et al.) are continuing. Antarctic total column ozone in September and October have continued to be 40–50 percent lower than pre-ozone-hole values since the 1990s.
A gradual trend toward "healing" was reported in 2016.
In 2017, NASA announced that the ozone hole was the weakest since 1988 because of warm stratospheric conditions. It is expected to recover around 2070.
The amount lost is more variable year-to-year in the
Arctic
The Arctic (; . ) is the polar regions of Earth, polar region of Earth that surrounds the North Pole, lying within the Arctic Circle. The Arctic region, from the IERS Reference Meridian travelling east, consists of parts of northern Norway ( ...
than in the Antarctic. The greatest Arctic declines are in the winter and spring, reaching up to 30 percent when the stratosphere is coldest.
Reactions that take place on polar stratospheric clouds (PSCs) play an important role in enhancing ozone depletion. PSCs form more readily in the extreme cold of the Arctic and Antarctic stratosphere. This is why ozone holes first formed, and are deeper, over Antarctica. Early models failed to take PSCs into account and predicted a gradual global depletion, which is why the sudden Antarctic ozone hole was such a surprise to many scientists.
It is more accurate to speak of ozone depletion in middle latitudes rather than holes. Total column ozone declined below pre-1980 values between 1980 and 1996 for mid-latitudes. In the northern mid-latitudes, it then increased from the minimum value by about two percent from 1996 to 2009 as regulations took effect and the amount of chlorine in the stratosphere decreased. In the Southern Hemisphere's mid-latitudes, total ozone remained constant over that time period. There are no significant trends in the tropics, largely because halogen-containing compounds have not had time to break down and release chlorine and bromine atoms at tropical latitudes.
Large volcanic eruptions have been shown to have substantial albeit uneven ozone-depleting effects, as observed with the 1991 eruption of Mt. Pinatubo in the Philippines.
Ozone depletion also explains much of the observed reduction in stratospheric and upper tropospheric temperatures.
The source of the warmth of the stratosphere is the absorption of UV radiation by ozone, hence reduced ozone leads to cooling. Some stratospheric cooling is also predicted from increases in
greenhouse gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. T ...
es such as and CFCs themselves; however, the ozone-induced cooling appears to be dominant.
Predictions of ozone levels remain difficult, but the precision of models' predictions of observed values and the agreement among different modeling techniques have increased steadily.
The World Meteorological Organization Global Ozone Research and Monitoring Project—Report No. 44 is strongly in favor of the
Montreal Protocol
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 ...
, but notes that a
UNEP
The United Nations Environment Programme (UNEP) is responsible for coordinating responses to environmental issues within the United Nations system. It was established by Maurice Strong, its first director, after the Declaration of the United Nati ...
1994 Assessment overestimated ozone loss for the 1994–1997 period.
Compounds in the atmosphere
CFCs and related compounds
Chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly Halogenation, halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatility (chemistry), volat ...
s (CFCs) and other halogenated ozone-depleting substances (ODS) are mainly responsible for man-made chemical ozone depletion. The total amount of effective halogens (chlorine and bromine) in the stratosphere can be calculated and are known as the
equivalent effective stratospheric chlorine (EESC).
CFCs as refrigerants were invented by
Thomas Midgley Jr. in the 1930s. They were used in
air conditioning
Air conditioning, often abbreviated as A/C (US) or air con (UK), is the process of removing heat from an enclosed space to achieve a more comfortable interior temperature, and in some cases, also controlling the humidity of internal air. Air c ...
and cooling units, as
aerosol spray propellants prior to the 1970s, and in the cleaning processes of delicate electronic equipment. They also occur as by-products of some chemical processes. No significant natural sources have ever been identified for these compounds—their presence in the atmosphere is due almost entirely to human manufacture. As mentioned above, when such ozone-depleting chemicals reach the stratosphere, they are dissociated by ultraviolet light to release chlorine atoms. The chlorine atoms act as a
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, and each can break down tens of thousands of ozone molecules before being removed from the stratosphere. Given the longevity of CFC molecules, recovery times are measured in decades. It is calculated that a CFC molecule takes an average of about five to seven years to go from the ground level up to the upper atmosphere, and it can stay there for about a century, destroying up to one hundred thousand ozone molecules during that time.
1,1,1-Trichloro-2,2,2-trifluoroethane, also known as CFC-113a, is one of four man-made chemicals newly discovered in the atmosphere by a team at the University of East Anglia. CFC-113a is the only known
CFC whose abundance in the atmosphere is still growing. Its source remains a mystery, but illegal manufacturing is suspected by some. CFC-113a seems to have been accumulating unabated since 1960. Between 2012 and 2017, concentrations of the gas jumped by 40 percent.
A study by an international team of researchers published in ''Nature'' found that since 2013 emissions that are predominately from north-eastern China have released large quantities of the banned chemical Chlorofluorocarbon-11 (CFC-11) into the atmosphere. Scientists estimate that without action, these CFC-11 emissions will delay the recovery of the planet's ozone hole by a decade.
Aluminum oxide
Satellite
A satellite or an artificial satellite is an object, typically a spacecraft, placed into orbit around a celestial body. They have a variety of uses, including communication relay, weather forecasting, navigation ( GPS), broadcasting, scient ...
s burning up upon re-entry into Earth's atmosphere produce
aluminum oxide
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly ...
(Al
2O
3)
nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
s that endure in the atmosphere for decades.
[ Estimates for 2022 alone were ~17 metric tons (~30kg of nanoparticles per ~250kg satellite).][ Increasing populations of ]satellite constellation
A satellite constellation is a group of artificial satellites working together as a system. Unlike a single satellite, a constellation can provide permanent global or near-global pass (spaceflight), coverage, such that at any time everywhere on E ...
s can eventually lead to significant ozone depletion.
Very short-lived substances (VSLS)
" Very short-lived substances" are a class of ozone-depleting chemicals, allowed by the Montreal Protocol
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 ...
, that degrade in under 6 months.bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
-based chemicals generated by seaweed and phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
, but 10% are manmade, for example dichloromethane.
Computer modeling
Scientists have attributed ozone depletion to the increase of man-made (anthropogenic
Anthropogenic ("human" + "generating") is an adjective that may refer to:
* Anthropogeny, the study of the origins of humanity
Anthropogenic may also refer to things that have been generated by humans, as follows:
* Human impact on the enviro ...
) halogen compounds from CFCs by combining observational data with computer models. These complex chemistry transport models (e.g. SLIMCAT, CLaMS
Clam is a common name for several kinds of bivalve mollusc. The word is often applied only to those that are deemed edible and live as infauna, spending most of their lives halfway buried in the sand of the sea floor or riverbeds. Clams h ...
—Chemical Lagrangian Model of the Stratosphere) work by combining measurements of chemicals and meteorological fields with chemical reaction rate constants. They identify key chemical reactions and transport processes that bring CFC photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
products into contact with ozone.
Ozone hole and its causes
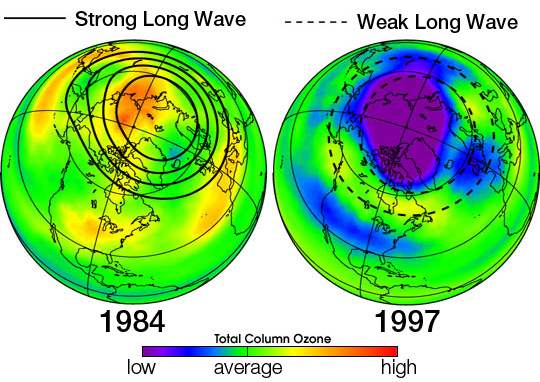 The Antarctic ozone hole is an area of the Antarctic stratosphere in which the recent ozone levels have dropped to as low as 33 percent of their pre-1975 values. The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container. Within this polar vortex, over 50 percent of the lower stratospheric ozone is destroyed during the Antarctic spring.
As explained above, the primary cause of ozone depletion is the presence of chlorine-containing source gases (primarily CFCs and related halocarbons). In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is substantially enhanced in the presence of polar stratospheric clouds (PSCs).
These polar stratospheric clouds form during winter, in the extreme cold. Polar winters are dark, consisting of three months without solar radiation (sunlight). The lack of sunlight contributes to a decrease in temperature and the polar vortex traps and chills the air. Temperatures are around or below −80 °C. These low temperatures form cloud particles. There are three types of PSC clouds—nitric acid trihydrate clouds, slowly cooling water-ice clouds, and rapid cooling water-ice (nacreous) clouds—provide surfaces for chemical reactions whose products will, in the spring lead to ozone destruction.
The photochemical processes involved are complex but well understood. The key observation is that, ordinarily, most of the chlorine in the stratosphere resides in "reservoir" compounds, primarily chlorine nitrate () as well as stable end products such as HCl. The formation of end products essentially removes Cl from the ozone depletion process. Reservoir compounds sequester Cl, which can later be made available via absorption of light at wavelengths shorter than 400 nm. During the Antarctic winter and spring, reactions on the surface of the polar stratospheric cloud particles convert these "reservoir" compounds into reactive free radicals (Cl and ClO). Denitrification is the process by which the clouds remove from the stratosphere by converting it to nitric acid in PSC particles, which then are lost by sedimentation. This prevents newly formed ClO from being converted back into .
The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring. During winter, even though PSCs are at their most abundant, there is no light over the pole to drive chemical reactions. During the spring, however, sunlight returns and provides energy to drive photochemical reactions and melt the polar stratospheric clouds, releasing considerable ClO, which drives the hole mechanism. Further warming temperatures near the end of spring break up the vortex around mid-December. As warm, ozone and -rich air flows in from lower latitudes, the PSCs are destroyed, the enhanced ozone depletion process shuts down, and the ozone hole closes.
Most of the ozone that is destroyed is in the lower stratosphere, in contrast to the much smaller ozone depletion through homogeneous gas-phase reactions, which occurs primarily in the upper stratosphere.
The Antarctic ozone hole is an area of the Antarctic stratosphere in which the recent ozone levels have dropped to as low as 33 percent of their pre-1975 values. The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container. Within this polar vortex, over 50 percent of the lower stratospheric ozone is destroyed during the Antarctic spring.
As explained above, the primary cause of ozone depletion is the presence of chlorine-containing source gases (primarily CFCs and related halocarbons). In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is substantially enhanced in the presence of polar stratospheric clouds (PSCs).
These polar stratospheric clouds form during winter, in the extreme cold. Polar winters are dark, consisting of three months without solar radiation (sunlight). The lack of sunlight contributes to a decrease in temperature and the polar vortex traps and chills the air. Temperatures are around or below −80 °C. These low temperatures form cloud particles. There are three types of PSC clouds—nitric acid trihydrate clouds, slowly cooling water-ice clouds, and rapid cooling water-ice (nacreous) clouds—provide surfaces for chemical reactions whose products will, in the spring lead to ozone destruction.
The photochemical processes involved are complex but well understood. The key observation is that, ordinarily, most of the chlorine in the stratosphere resides in "reservoir" compounds, primarily chlorine nitrate () as well as stable end products such as HCl. The formation of end products essentially removes Cl from the ozone depletion process. Reservoir compounds sequester Cl, which can later be made available via absorption of light at wavelengths shorter than 400 nm. During the Antarctic winter and spring, reactions on the surface of the polar stratospheric cloud particles convert these "reservoir" compounds into reactive free radicals (Cl and ClO). Denitrification is the process by which the clouds remove from the stratosphere by converting it to nitric acid in PSC particles, which then are lost by sedimentation. This prevents newly formed ClO from being converted back into .
The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring. During winter, even though PSCs are at their most abundant, there is no light over the pole to drive chemical reactions. During the spring, however, sunlight returns and provides energy to drive photochemical reactions and melt the polar stratospheric clouds, releasing considerable ClO, which drives the hole mechanism. Further warming temperatures near the end of spring break up the vortex around mid-December. As warm, ozone and -rich air flows in from lower latitudes, the PSCs are destroyed, the enhanced ozone depletion process shuts down, and the ozone hole closes.
Most of the ozone that is destroyed is in the lower stratosphere, in contrast to the much smaller ozone depletion through homogeneous gas-phase reactions, which occurs primarily in the upper stratosphere.
Effects
Since the ozone layer absorbs UVB ultraviolet light from the sun, ozone layer depletion increases surface UVB levels (all else equal), which could lead to damage, including an increase in skin cancer
Skin cancers are cancers that arise from the Human skin, skin. They are due to the development of abnormal cells (biology), cells that have the ability to invade or metastasis, spread to other parts of the body. It occurs when skin cells grow ...
. This was the reason for the Montreal Protocol. Although decreases in stratospheric ozone are well-tied to CFCs and increases in surface UVB, there is no direct observational evidence linking ozone depletion to higher incidence of skin cancer and eye damage in human beings. This is partly because UVA, which has also been implicated in some forms of skin cancer, is not absorbed by ozone, and because it is nearly impossible to control statistics for lifestyle changes over time. Ozone depletion may also influence wind patterns.
Increased UV
Ozone, while a minority constituent in Earth's atmosphere, is responsible for most of the absorption of UVB radiation. The amount of UVB radiation that penetrates through the ozone layer decreases exponentially with the slant-path thickness and density of the layer. When stratospheric ozone levels decrease, higher levels of UVB reach the Earth's surface.Quito
Quito (; ), officially San Francisco de Quito, is the capital city, capital and second-largest city of Ecuador, with an estimated population of 2.8 million in its metropolitan area. It is also the capital of the province of Pichincha Province, P ...
; the WHO considers 11 as an extreme index and a great risk to health. The report concluded that depleted ozone levels around the mid-latitudes of the planet are already endangering large populations in these areas. Later, the CONIDA, the Peruvian Space Agency, published its own study, which yielded almost the same findings as the Ecuadorian study.
Biological effects
The main public concern regarding the ozone hole has been the effects of increased surface UV radiation on human health. So far, ozone depletion in most locations has been typically a few percent and, as noted above, no direct evidence of health damage is available in most latitudes. If the high levels of depletion seen in the ozone hole were to be common across the globe, the effects could be substantially more dramatic. As the ozone hole over Antarctica has in some instances grown so large as to affect parts of Australia
Australia, officially the Commonwealth of Australia, is a country comprising mainland Australia, the mainland of the Australia (continent), Australian continent, the island of Tasmania and list of islands of Australia, numerous smaller isl ...
, New Zealand
New Zealand () is an island country in the southwestern Pacific Ocean. It consists of two main landmasses—the North Island () and the South Island ()—and List of islands of New Zealand, over 600 smaller islands. It is the List of isla ...
, Chile
Chile, officially the Republic of Chile, is a country in western South America. It is the southernmost country in the world and the closest to Antarctica, stretching along a narrow strip of land between the Andes, Andes Mountains and the Paci ...
, Argentina
Argentina, officially the Argentine Republic, is a country in the southern half of South America. It covers an area of , making it the List of South American countries by area, second-largest country in South America after Brazil, the fourt ...
, and South Africa
South Africa, officially the Republic of South Africa (RSA), is the Southern Africa, southernmost country in Africa. Its Provinces of South Africa, nine provinces are bounded to the south by of coastline that stretches along the Atlantic O ...
, environmentalists have been concerned that the increase in surface UV could be significant. Excessive ultraviolet radiation (UVR) has reducing effects on the rates of photosynthesis and growth of benthic diatom
A diatom (Neo-Latin ''diatoma'') is any member of a large group comprising several Genus, genera of algae, specifically microalgae, found in the oceans, waterways and soils of the world. Living diatoms make up a significant portion of Earth's B ...
communities (microalgae species that increase water quality and are pollution resistant) that are present in shallow freshwater. Ozone depletion not only affects human health but also has a profound impact on biodiversity. It damages plants and trees at the cellular level, affecting their growth, vitality, photosynthesis, water balance, and defense mechanisms against pests and diseases. This sets off a cascade of ecological impacts, harming soil microbes, insects, wildlife, and entire ecosystems.
Ozone depletion would magnify all of the effects of UV on human health, both positive (including production of vitamin D) and negative (including sunburn, skin cancer, and cataracts). In addition, increased surface UV leads to increased tropospheric ozone, which is a health risk to humans.
Basal and squamous cell carcinomas
The most common forms of skin cancer in humans, basal and squamous
Epithelium or epithelial tissue is a thin, continuous, protective layer of cells with little extracellular matrix. An example is the epidermis, the outermost layer of the skin. Epithelial ( mesothelial) tissues line the outer surfaces of man ...
cell carcinomas, have been strongly linked to UV-B exposure. The mechanism by which UVB induces these cancers is well understood—absorption of UV-B radiation causes the pyrimidine bases in the DNA molecule to form dimers, resulting in transcription errors when the DNA replicates. These cancers are relatively mild and rarely fatal, although the treatment of squamous cell carcinoma sometimes requires extensive reconstructive surgery. By combining epidemiological data with results of animal studies, scientists have estimated that every one percent decrease in long-term stratospheric ozone would increase the incidence of these cancers by 2%.
Melanoma
Another form of skin cancer, Melanoma
Melanoma is the most dangerous type of skin cancer; it develops from the melanin-producing cells known as melanocytes. It typically occurs in the skin, but may rarely occur in the mouth, intestines, or eye (uveal melanoma). In very rare case ...
, is much less common but far more dangerous, being lethal in about 15–20 percent of the cases diagnosed. The relationship between melanoma and ultraviolet exposure is not yet fully understood, but it appears that both UV-B and UV-A are involved. Because of this uncertainty, it is difficult to estimate the effect of ozone depletion on melanoma incidence. One study showed that a 10 percent increase in UV-B radiation was associated with a 19 percent increase in melanomas for men and 16 percent for women. A study of people in Punta Arenas
Punta Arenas (, historically known as Sandy Point in English) is the capital List of cities in Chile, city of Chile's southernmost Regions of Chile, region, Magallanes Region, Magallanes and Antarctica Chilena. Although officially renamed as ...
, at the southern tip of Chile
Chile, officially the Republic of Chile, is a country in western South America. It is the southernmost country in the world and the closest to Antarctica, stretching along a narrow strip of land between the Andes, Andes Mountains and the Paci ...
, showed a 56 percent increase in melanoma and a 46 percent increase in non-melanoma skin cancer over a period of seven years, along with decreased ozone and increased UVB levels.
Cortical cataracts
Epidemiological studies suggest an association between ocular cortical cataracts and UV-B exposure, using crude approximations of exposure and various cataract assessment techniques. A detailed assessment of ocular exposure to UV-B was carried out in a study on Chesapeake Bay Watermen, where increases in average annual ocular exposure were associated with increasing risk of cortical opacity. In this highly exposed group of predominantly white males, the evidence linking cortical opacities to sunlight exposure was the strongest to date. Based on these results, ozone depletion is predicted to cause hundreds of thousands of additional cataracts by 2050.
Increased tropospheric ozone
Increased surface UV leads to increased tropospheric ozone. Ground-level ozone
Ground-level ozone (), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the atmosphere of Earth, Earth's atmosphere), with an average concentration of 20–30 parts per billion by vo ...
is generally recognized to be a health risk, as ozone is toxic due to its strong oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ''electr ...
properties. The risks are particularly high for young children, the elderly, and those with asthma or other respiratory difficulties. At this time, ozone at ground level is produced mainly by the action of UV radiation on combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
gases from vehicle exhausts.
Increased production of vitamin D
Vitamin D
Vitamin D is a group of structurally related, fat-soluble compounds responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, along with numerous other biological functions. In humans, the most important compo ...
is produced in the skin by ultraviolet light. Thus, higher UVB exposure raises human vitamin D in those deficient in it. Recent research (primarily since the Montreal Protocol) shows that many humans have less than optimal vitamin D levels. In particular, in the U.S. population, the lowest quarter of vitamin D (<17.8 ng/ml) were found using information from the National Health and Nutrition Examination Survey to be associated with an increase in all-cause mortality in the general population. While blood level of vitamin D in excess of 100 ng/ml appear to raise blood calcium excessively and to be associated with higher mortality, the body has mechanisms that prevent sunlight from producing vitamin D in excess of the body's requirements.
Effects on animals
A November 2011 report by scientists at the Institute of Zoology in London, England found that whale
Whales are a widely distributed and diverse group of fully Aquatic animal, aquatic placental mammal, placental marine mammals. As an informal and Colloquialism, colloquial grouping, they correspond to large members of the infraorder Cetacea ...
s off the coast of California have shown a sharp rise in sun damage, and these scientists "fear that the thinning ozone layer is to blame". The study photographed and took skin biopsies from over 150 whales in the Gulf of California and found "widespread evidence of epidermal damage commonly associated with acute and severe sunburn", having cells that form when the DNA is damaged by UV radiation. The findings suggest "rising UV levels as a result of ozone depletion are to blame for the observed skin damage, in the same way that human skin cancer rates have been on the increase in recent decades." Apart from whales many other animals such as dogs, cats, sheep and terrestrial ecosystems also suffer the negative effects of increased UV-B radiations.
Effects on crops
An increase of UV radiation would be expected to affect crops. A number of economically important species of plants, such as rice
Rice is a cereal grain and in its Domestication, domesticated form is the staple food of over half of the world's population, particularly in Asia and Africa. Rice is the seed of the grass species ''Oryza sativa'' (Asian rice)—or, much l ...
, depend on cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteri ...
residing on their roots for the retention of nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
. Cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteri ...
are sensitive to UV radiation and would be affected by its increase. "Despite mechanisms to reduce or repair the effects of increased ultraviolet radiation, plants have a limited ability to adapt to increased levels of UVB, therefore plant growth can be directly affected by UVB radiation."
Effects on plant life
Over the years, the Arctic ozone layer has depleted severely. As a consequence species that live above the snow cover or in areas where snow has melted abundantly, due to hot temperatures, are negatively impacted due to UV radiation that reaches the ground. Depletion of the ozone layer and allowing excess UVB radiation would initially be assumed to increase damage to plant DNA. Reports have found that when plants are exposed to UVB radiation similar to stratospheric ozone depletion, there was no significant change in plant height or leaf mass, but showed a response in shoot biomass and leaf area with a small decrease. However, UVB radiation has been shown to decrease quantum yield of photosystem II. UVB damage only occurs under extreme exposure, and most plants also have UVB absorbing flavonoids which allow them to acclimatize to the radiation present. Plants experience different levels of UV radiation throughout the day. It is known that they are able to shift the levels and types of UV sunscreens (i.e. flavonoids), that they contain, throughout the day. This allows them to increase their protection against UV radiation. Plants that have been affected by radiation throughout development are more affected by the inability to intercept light with a larger leaf area than having photosynthetic systems compromised. Damage from UVB radiation is more likely to be significant on species interactions than on plants themselves.
Another significant impact of ozone depletion on plant life is the stress experienced by plants when exposed to UV radiation. This can cause a decrease in plant growth and an increase in oxidative stress, due to the production of nitric oxide and hydrogen peroxide. In areas where substantial ozone depletion has occurred, increased UV-B radiation reduces terrestrial plant productivity (and likewise carbon sequestration) by about 6%.
Moreover, if plants are exposed to high levels of UV radiation, it can elicit the production of harmful volatile organic compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapor pressure at room temperature. They are common and exist in a variety of settings and products, not limited to Indoor mold, house mold, Upholstery, upholstered furnitur ...
s, like isoprenes. The emission of isoprenes into the air, by plants, can severely impact the environment by adding to air pollution and increasing the amount of carbon in the atmosphere, ultimately contributing to climate change.
Public policy
 The full extent of the damage that CFCs have caused to the ozone layer is not known and will not be known for decades; however, marked decreases in column ozone have already been observed. The Montreal and Vienna conventions were installed long before a scientific consensus was established or important uncertainties in the science field were being resolved.
The full extent of the damage that CFCs have caused to the ozone layer is not known and will not be known for decades; however, marked decreases in column ozone have already been observed. The Montreal and Vienna conventions were installed long before a scientific consensus was established or important uncertainties in the science field were being resolved.United States National Academy of Sciences
The National Academy of Sciences (NAS) is a United States nonprofit, non-governmental organization. NAS is part of the National Academies of Sciences, Engineering, and Medicine, along with the National Academy of Engineering (NAE) and the Nati ...
concluded that credible scientific evidence supported the ozone depletion hypothesisFreon
Freon ( ) is a registered trademark of the Chemours Company and generic descriptor for a number of halocarbon products. They are stable, nonflammable, low toxicity gases or liquids which have generally been used as refrigerants and as aerosol p ...
was set to expire in 1979. The United States banned the use of CFCs in aerosol cans in 1978.DuPont
Dupont, DuPont, Du Pont, duPont, or du Pont may refer to:
People
* Dupont (surname) Dupont, also spelled as DuPont, duPont, Du Pont, or du Pont is a French surname meaning "of the bridge", historically indicating that the holder of the surname re ...
Canada closed its CFC facility.
The U.S. government's attitude began to change again in 1983, when William Ruckelshaus
William Doyle Ruckelshaus (July 24, 1932 – November 27, 2019) was an American attorney and government official.
Ruckelshaus served in the Indiana House of Representatives from 1966 to 1968, and was the United States Assistant Attorney General ...
replaced Anne M. Burford as Administrator of the United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent agency of the United States government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it began operation on De ...
(EPA). Under Ruckelshaus and his successor, Lee Thomas, the EPA pushed for an international approach to halocarbon regulations. In 1985 twenty nations, including most of the major CFC producers, signed the Vienna Convention for the Protection of the Ozone Layer, which established a framework for negotiating international regulations on ozone-depleting substances. That same year, the discovery of the Antarctic ozone hole was announced, causing a revival in public attention to the issue.
In 1987, representatives from 43 nations signed the Montreal Protocol
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 ...
. Meanwhile, the halocarbon industry shifted its position and started supporting a protocol to limit CFC production. However, this shift was uneven with DuPont acting more quickly than its European counterparts. DuPont may have feared court action related to increased skin cancer, especially as the EPA had published a study in 1986 claiming that an additional 40 million cases and 800,000 cancer deaths were to be expected in the U.S. in the next 88 years. The EU shifted its position as well after Germany gave up its defence of the CFC industry and started supporting moves towards regulation. Government and industry in France and the UK tried to defend their CFC producing industries even after the Montreal Protocol had been signed.methyl bromide
Bromomethane, commonly known as methyl bromide, is an organobromine compound with chemical formula, formula Carbon, CHydrogen, H3Bromine, Br. This colorless, odorless, nonflammable gas is Bromine cycle, produced both industrially and biologically ...
(MeBr), a fumigant used primarily in agricultural production, was added to the list of controlled substances. For all substances controlled under the protocol, phaseout schedules were delayed for less developed ('Article 5(1)') countries, and phaseout in these countries was supported by transfers of expertise, technology, and money from non-Article 5(1) Parties to the Protocol. Additionally, exemptions from the agreed schedules could be applied for under the Essential Use Exemption (EUE) process for substances other than methyl bromide and under the Critical Use Exemption (CUE) process for methyl bromide.
Civil society, including especially non-governmental organizations (NGOs), played critical roles at all stages of policy development leading to the Vienna Conference, the Montreal Protocol, and in assessing compliance afterwards. The major companies claimed that no alternatives to HFC existed.propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum ref ...
and butane
Butane () is an alkane with the formula C4H10. Butane exists as two isomers, ''n''-butane with connectivity and iso-butane with the formula . Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at ro ...
, and in 1992 came to the attention of the NGO Greenpeace. Greenpeace called it "Greenfreeze". The NGO then worked successfully first with a small and struggling company to market an appliance beginning in Europe, then Asia and later Latin America, receiving a 1997 UNEP award.HCFC
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatile derivatives of methane, ethane, ...
s), although concerns remain regarding HCFCs also. In some applications, hydrofluorocarbons ( HFCs) were being used to replace CFCs. HFCs, which contain no chlorine or bromine, do not contribute to ozone depletion although they are potent greenhouse gases. The best known of these compounds is probably HFC-134a ( R-134a), which in the United States has largely replaced CFC-12 ( R-12) in automobile air conditioners. In laboratory analytics (a former "essential" use) the ozone depleting substances can be replaced with other solvents. Chemical companies like Du Pont, whose representatives disparaged Greenfreeze as "that German technology," maneuvered the EPA to block the technology in the U.S. until 2011. Ben & Jerry's of Unilever and General Electric, spurred by Greenpeace, had expressed formal interest in 2008 which figured in the EPA's final approval.climate change
Present-day climate change includes both global warming—the ongoing increase in Global surface temperature, global average temperature—and its wider effects on Earth's climate system. Climate variability and change, Climate change in ...
. The reduction of the radiative forcing due to ODS probably masked the true level of climate change effects of other greenhouse gases, and was responsible for the "slow down" of global warming from the mid-90s. Policy decisions in one arena affect the costs and effectiveness of environmental improvements in the other.
ODS requirements in the marine industry
The IMO has amended MARPOL Annex VI Regulation 12 regarding ozone depleting substances. As from July 1, 2010, all vessels where MARPOL Annex VI is applicable should have a list of equipment using ozone depleting substances. The list should include the name of ODS, type and location of equipment, quantity in kg and date. All changes since that date should be recorded in an ODS Record book on board recording all intended or unintended releases to the atmosphere. Furthermore, new ODS supply or landing to shore facilities should be recorded as well.
Prospects of ozone depletion
 Since the adoption and strengthening of the
Since the adoption and strengthening of the Montreal Protocol
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 ...
has led to reductions in the emissions of CFCs, atmospheric concentrations of the most-significant compounds have been declining. These substances are being gradually removed from the atmosphere; since peaking in 1994, the Effective Equivalent Chlorine (EECl) level in the atmosphere had dropped about 10 percent by 2008. The decrease in ozone-depleting chemicals has also been significantly affected by a decrease in bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
-containing chemicals. The data suggest that substantial natural sources exist for atmospheric methyl bromide
Bromomethane, commonly known as methyl bromide, is an organobromine compound with chemical formula, formula Carbon, CHydrogen, H3Bromine, Br. This colorless, odorless, nonflammable gas is Bromine cycle, produced both industrially and biologically ...
().nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room te ...
(), which is not covered by the Montreal Protocol, has become the most highly emitted ozone-depleting substance and is expected to remain so throughout the 21st century.
According to the IPCC Sixth Assessment Report, global stratospheric ozone levels experienced rapid decline in the 1970s and 1980s and have since been increasing, but have not reached preindustrial levels. Although considerable variability is expected from year to year, including in polar regions where depletion is largest, the ozone layer is expected to continue recovering in coming decades due to declining ozone-depleting substance concentrations, assuming full compliance with the Montreal Protocol.[ In 2019, the ozone hole was at its smallest in the previous thirty years, due to the warmer polar stratosphere weakening the polar vortex. In September 2023, the Antarctic ozone hole was one of the largest on record, at 26 million square kilometers. The anomalously large ozone loss may have been a result of the 2022 Tonga volcanic eruption.
According to a 2023 United Nations assessment, the ozone layer is on track to recover to 1980 levels by around 2066 over Antarctica, by 2045 over the Arctic, and by 2040 for the rest of the world, assuming current regulations remain in place.]
Research history
The basic physical and chemical processes that lead to the formation of an ozone layer in the Earth's stratosphere were discovered by Sydney Chapman in 1930. Short-wavelength UV radiation splits an oxygen () molecule into two oxygen (O) atoms, which then combine with other oxygen molecules to form ozone. Ozone is removed when an oxygen atom and an ozone molecule "recombine" to form two oxygen molecules, i.e. O + → 2. In the 1950s, David Bates and Marcel Nicolet presented evidence that various free radicals, in particular hydroxyl (OH) and nitric oxide (NO), could catalyze this recombination reaction, reducing the overall amount of ozone. These free radicals were known to be present in the stratosphere, and so were regarded as part of the natural balance—it was estimated that in their absence, the ozone layer would be about twice as thick as it currently is.
In 1970 Paul Crutzen
Paul Jozef Crutzen (; 3 December 1933 – 28 January 2021) was a Dutch meteorologist and atmospheric chemistry, atmospheric chemist. In 1995, he was awarded the Nobel Prize in Chemistry alongside Mario Molina and F. Sherwood Rowland, Frank Sherw ...
pointed out that emissions of nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room te ...
(), a stable, long-lived gas produced by soil bacteria, from the Earth's surface could affect the amount of nitric oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes den ...
(NO) in the stratosphere. Crutzen showed that nitrous oxide lives long enough to reach the stratosphere, where it is converted into NO. Crutzen then noted that increasing use of fertilizers
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrition, plant nutrients. Fertilizers may be distinct from Liming (soil), liming materials or other non- ...
might have led to an increase in nitrous oxide emissions over the natural background, which would in turn result in an increase in the amount of NO in the stratosphere. Thus human activity could affect the stratospheric ozone layer. In the following year, Crutzen and (independently) Harold Johnston suggested that NO emissions from supersonic passenger aircraft, which would fly in the lower stratosphere, could also deplete the ozone layer. However, more recent analysis in 1995 by David W. Fahey, an atmospheric scientist at the National Oceanic and Atmospheric Administration
The National Oceanic and Atmospheric Administration (NOAA ) is an American scientific and regulatory agency charged with Weather forecasting, forecasting weather, monitoring oceanic and atmospheric conditions, Hydrography, charting the seas, ...
, found that the drop in ozone would be from 1–2 percent if a fleet of 500 supersonic passenger aircraft were operated. This, Fahey expressed, would not be a showstopper for advanced supersonic passenger aircraft development.
Rowland–Molina hypothesis
In 1974 Frank Sherwood Rowland, Chemistry Professor at the University of California at Irvine, and his postdoctoral associate Mario J. Molina suggested that long-lived organic halogen compounds, such as CFCs, might behave in a similar fashion as Crutzen had proposed for nitrous oxide. James Lovelock had recently discovered, during a cruise in the South Atlantic in 1971, that almost all of the CFC compounds manufactured since their invention in 1930 were still present in the atmosphere. Molina and Rowland concluded that, like , the CFCs would reach the stratosphere where they would be dissociated by UV light, releasing chlorine atoms. A year earlier, Richard Stolarski and Ralph Cicerone at the University of Michigan had shown that Cl is even more efficient than NO at catalyzing the destruction of ozone. Similar conclusions were reached by Michael McElroy and Steven Wofsy at Harvard University
Harvard University is a Private university, private Ivy League research university in Cambridge, Massachusetts, United States. Founded in 1636 and named for its first benefactor, the History of the Puritans in North America, Puritan clergyma ...
. Neither group, however, had realized that CFCs were a potentially large source of stratospheric chlorine—instead, they had been investigating the possible effects of HCl emissions from the Space Shuttle
The Space Shuttle is a retired, partially reusable launch system, reusable low Earth orbital spacecraft system operated from 1981 to 2011 by the U.S. National Aeronautics and Space Administration (NASA) as part of the Space Shuttle program. ...
, which are very much smaller.
The Rowland–Molina hypothesis was strongly disputed by representatives of the aerosol and halocarbon industries. The Chair of the Board of DuPont
Dupont, DuPont, Du Pont, duPont, or du Pont may refer to:
People
* Dupont (surname) Dupont, also spelled as DuPont, duPont, Du Pont, or du Pont is a French surname meaning "of the bridge", historically indicating that the holder of the surname re ...
was quoted as saying that ozone depletion theory is "a science fiction tale ... a load of rubbish ... utter nonsense".Robert Abplanalp
Robert Henry Abplanalp, ( KHS) (April 4, 1922 – August 30, 2003) was an American inventor and engineer who invented the modern form of the aerosol spray valve, the founder of Precision Valve Corporation, a Republican political activist, and ...
, the President of Precision Valve Corporation (and inventor of the first practical aerosol spray can valve), wrote to the Chancellor of UC Irvine
UC may refer to:
Education
In the United States
* University of California system
* University of Charleston, West Virginia
* University of Chicago, Illinois
* University of Cincinnati, Ohio
* Upsala College, East Orange, New Jersey (''defunct ...
to complain about Rowland's public statements. Nevertheless, within three years most of the basic assumptions made by Rowland and Molina were confirmed by laboratory measurements and by direct observation in the stratosphere. The concentrations of the source gases (CFCs and related compounds) and the chlorine reservoir species (HCl and ) were measured throughout the stratosphere, and demonstrated that CFCs were indeed the major source of stratospheric chlorine, and that nearly all of the CFCs emitted would eventually reach the stratosphere. Even more convincing was the measurement, by James G. Anderson and collaborators, of chlorine monoxide (ClO) in the stratosphere. ClO is produced by the reaction of Cl with ozone—its observation thus demonstrated that Cl radicals not only were present in the stratosphere but also were actually involved in destroying ozone. McElroy and Wofsy extended the work of Rowland and Molina by showing that bromine atoms were even more effective catalysts for ozone loss than chlorine atoms and argued that the brominated organic compounds known as halons, widely used in fire extinguishers, were a potentially large source of stratospheric bromine. In 1976 the United States National Academy of Sciences
The National Academy of Sciences (NAS) is a United States nonprofit, non-governmental organization. NAS is part of the National Academies of Sciences, Engineering, and Medicine, along with the National Academy of Engineering (NAE) and the Nati ...
released a report concluding that the ozone depletion hypothesis was strongly supported by the scientific evidence. In response the United States, Canada and Norway banned the use of CFCs in aerosol spray cans in 1978. Early estimates were that, if CFC production continued at 1977 levels, the total atmospheric ozone would after a century or so reach a steady state, 15 to 18 percent below normal levels. By 1984, when better evidence on the speed of critical reactions was available, this estimate was changed to 5 to 9 percent steady-state depletion.Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
for their work on stratospheric ozone.
Antarctic ozone hole
The discovery of the Antarctic "ozone hole" by British Antarctic Survey
The British Antarctic Survey (BAS) is the United Kingdom's national polar research institute. It has a dual purpose, to conduct polar science, enabling better understanding of list of global issues, global issues, and to provide an active prese ...
scientists Farman, Gardiner Gardiner may refer to:
Places
Settlements
;Canada
* Gardiner, Ontario
;United States
* Gardiner, Maine
* Gardiner, Montana
* Gardiner (town), New York
** Gardiner (CDP), New York
* Gardiner, Oregon
* Gardiner, Washington
* West Gardiner, ...
and Shanklin
Shanklin () is a seaside resort town and civil parishes in England, civil parish on the Isle of Wight, England, located on Sandown Bay. Shanklin is the southernmost of three settlements which occupy the bay, and is close to Lake, Isle of Wight, ...
(first reported in a paper in ''Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
'' in May 1985) came as a shock to the scientific community, because the observed decline in polar ozone was far larger than had been anticipated.south pole
The South Pole, also known as the Geographic South Pole or Terrestrial South Pole, is the point in the Southern Hemisphere where the Earth's rotation, Earth's axis of rotation meets its surface. It is called the True South Pole to distinguish ...
were becoming available at the same time.software
Software consists of computer programs that instruct the Execution (computing), execution of a computer. Software also includes design documents and specifications.
The history of software is closely tied to the development of digital comput ...
was rerun without the flags, the ozone hole was seen as far back as 1976.
Susan Solomon, an atmospheric chemist at the National Oceanic and Atmospheric Administration
The National Oceanic and Atmospheric Administration (NOAA ) is an American scientific and regulatory agency charged with Weather forecasting, forecasting weather, monitoring oceanic and atmospheric conditions, Hydrography, charting the seas, ...
(NOAA), proposed that chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s on polar stratospheric clouds (PSCs) in the cold Antarctic
The Antarctic (, ; commonly ) is the polar regions of Earth, polar region of Earth that surrounds the South Pole, lying within the Antarctic Circle. It is antipodes, diametrically opposite of the Arctic region around the North Pole.
The Antar ...
stratosphere
The stratosphere () is the second-lowest layer of the atmosphere of Earth, located above the troposphere and below the mesosphere. The stratosphere is composed of stratified temperature zones, with the warmer layers of air located higher ...
caused a massive, though localized and seasonal, increase in the amount of chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
present in active, ozone-destroying forms. The polar stratospheric clouds in Antarctica are only formed at very low temperatures, as low as −80 °C, and early spring conditions. In such conditions the ice crystals
Ice crystals are solid water (known as ice) in crystal structure, symmetrical shapes including hexagonal crystal family, hexagonal columns, hexagonal plates, and dendrite (crystal), dendritic crystals. Ice crystals are responsible for various at ...
of the cloud provide a suitable surface for conversion of unreactive chlorine compounds into reactive chlorine compounds, which can easily deplete ozone.
Moreover, the polar vortex formed over Antarctica
Antarctica () is Earth's southernmost and least-populated continent. Situated almost entirely south of the Antarctic Circle and surrounded by the Southern Ocean (also known as the Antarctic Ocean), it contains the geographic South Pole. ...
is very tight and the reaction occurring on the surface of the cloud crystals is far different from when it occurs in atmosphere. These conditions have led to ozone hole formation in Antarctica. This hypothesis
A hypothesis (: hypotheses) is a proposed explanation for a phenomenon. A scientific hypothesis must be based on observations and make a testable and reproducible prediction about reality, in a process beginning with an educated guess o ...
was decisively confirmed, first by laboratory
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratories are found in a variety of settings such as schools ...
measurements and subsequently by direct measurements, from the ground and from high-altitude airplane
An airplane (American English), or aeroplane (Commonwealth English), informally plane, is a fixed-wing aircraft that is propelled forward by thrust from a jet engine, Propeller (aircraft), propeller, or rocket engine. Airplanes come in a vari ...
s, of very high concentrations of chlorine monoxide (ClO) in the Antarctic stratosphere.
Alternative hypotheses, which had attributed the ozone hole to variations in solar UV radiation
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of t ...
or to changes in atmospheric circulation patterns, were also tested and shown to be untenable.
Meanwhile, analysis of ozone measurements from the worldwide network of ground-based Dobson spectrophotometers led an international panel to conclude that the ozone layer was in fact being depleted, at all latitudes outside of the tropics.United Nations Environment Programme
The United Nations Environment Programme (UNEP) is responsible for coordinating responses to environmental issues within the United Nations system. It was established by Maurice Strong, its first director, after the Declaration of the United Nati ...
, under the auspices of the World Meteorological Organization, has sponsored a series of technical reports on the Scientific Assessment of Ozone Depletion, based on satellite measurements. The 2007 report showed that the hole in the ozone layer was recovering and the smallest it had been for about a decade.
A 2010 report found, "Over the past decade, global ozone and ozone in the Arctic and Antarctic regions is no longer decreasing but is not yet increasing. The ozone layer outside the Polar regions is projected to recover to its pre-1980 levels some time before the middle of this century. In contrast, the springtime ozone hole over the Antarctic is expected to recover much later."
In 2012, NOAA
The National Oceanic and Atmospheric Administration (NOAA ) is an American scientific and regulatory agency charged with forecasting weather, monitoring oceanic and atmospheric conditions, charting the seas, conducting deep-sea exploratio ...
and NASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the federal government of the United States, US federal government responsible for the United States ...
reported "Warmer air temperatures high above the Antarctic led to the second smallest season ozone hole in 20 years averaging 17.9 million square kilometres. The hole reached its maximum size for the season on Sept 22, stretching to 21.2 million square kilometres." A gradual trend toward "healing" was reported in 2016
Arctic ozone "mini-hole"
On March 3, 2005, the journal ''Nature'' published an article linking 2004's unusually large Arctic ozone hole to solar wind activity.
On March 15, 2011, a record ozone layer loss was observed, with about half of the ozone present over the Arctic having been destroyed.[ The change was attributed to increasingly cold winters in the Arctic stratosphere at an altitude of approximately , a change associated with global warming in a relationship that is still under investigation.]Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
'', which said that between December 2010 and March 2011 up to 80 percent of the ozone in the atmosphere at about above the surface was destroyed.
Tibet ozone hole
As winters that are colder are more affected, at times there is an ozone hole over Tibet. In 2006, a 2.5 million square kilometer ozone hole was detected over Tibet. Again in 2011, an ozone hole appeared over mountainous regions of Tibet
Tibet (; ''Böd''; ), or Greater Tibet, is a region in the western part of East Asia, covering much of the Tibetan Plateau and spanning about . It is the homeland of the Tibetan people. Also resident on the plateau are other ethnic groups s ...
, Xinjiang
Xinjiang,; , SASM/GNC romanization, SASM/GNC: Chinese postal romanization, previously romanized as Sinkiang, officially the Xinjiang Uygur Autonomous Region (XUAR), is an Autonomous regions of China, autonomous region of the China, People' ...
, Qinghai
Qinghai is an inland Provinces of China, province in Northwestern China. It is the largest provinces of China, province of China (excluding autonomous regions) by area and has the third smallest population. Its capital and largest city is Xin ...
and the Hindu Kush
The Hindu Kush is an mountain range in Central Asia, Central and South Asia to the west of the Himalayas. It stretches from central and eastern Afghanistan into northwestern Pakistan and far southeastern Tajikistan. The range forms the wester ...
, along with an unprecedented hole over the Arctic, though the Tibet one was far less intense than the ones over the Arctic or Antarctic.
Potential depletion by storm clouds
Research in 2012 showed that the same process that produces the ozone hole over Antarctica, occurs over summer storm clouds in the United States, and thus may be destroying ozone there as well.
Ozone hole over tropics
Physicist Qing-Bin Lu, of the University of Waterloo, claimed to have discovered a large, all-season ozone hole in the lower stratosphere over the tropics in July 2022. However, other researchers in the field refuted this claim, stating that the research was riddled with "serious errors and unsubstantiated assertions." According to Dr Paul Young, a lead author of the 2022 WMO/UNEP Scientific Assessment of Ozone Depletion, "The author's identification of a 'tropical ozone hole' is down to him looking at percentage changes in ozone, rather than absolute changes, with the latter being much more relevant for damaging UV reaching the surface." Specifically, Lu's work defines "ozone hole" as "an area with O3 loss in percent larger than 25%, with respect to the undisturbed O3 value when there were no significant CFCs in the stratosphere (~ in the 1960s)" instead of the general definition of 220 Dobson units or lower. Dr Marta Abalos Alvarez has added "Ozone depletion in the tropics is nothing new and is mainly due to the acceleration of the Brewer-Dobson circulation."
Depletion caused by wildfire smoke
Analyzing the atmospheric impacts of the 2019–2020 Australian bushfire season, scientists led by MIT researcher Susan Solomon found the smoke destroyed 3–5% of ozone in affected areas of the Southern Hemisphere. Smoke particles absorb hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
and act as a catalyst to create chlorine radicals that destroy ozone.
Ozone depletion and global warming
Among others, Robert Watson had a role in the science assessment and in the regulation efforts of ozone depletion and global warming Ozone depletion and climate change are environmental challenges whose connections have been explored and which have been compared and contrasted, for example in terms of global regulation, in various studies and books.
There is widespread scientifi ...
.[ Reiner Grundmannbr>''Technische Problemlösung, Verhandeln und umfassende Problemlösun''g, generic problem solving capability)]
in Gesellschaftliche Komplexität und kollektive Handlungsfähigkeit (Societys complexity and collective ability to act), ed. Schimank, U. (2000). Frankfurt/Main, Germany: Campus, pp. 154–182
book summary at the Max Planck Gesellschaft
. Prior to the 1980s, the EU, NASA, NAS, UNEP, WMO and the British government had dissenting scientific reports and Watson played a role in the process of unified assessments. Based on the experience with the ozone case, the IPCC started to work on a unified reporting and science assessment * The same radiative forcing that produces global warming is expected to cool the stratosphere.
* The same radiative forcing that produces global warming is expected to cool the stratosphere.watt
The watt (symbol: W) is the unit of Power (physics), power or radiant flux in the International System of Units (SI), equal to 1 joule per second or 1 kg⋅m2⋅s−3. It is used to quantification (science), quantify the rate of Work ...
s per square meter (W/m2).National Oceanic and Atmospheric Administration
The National Oceanic and Atmospheric Administration (NOAA ) is an American scientific and regulatory agency charged with Weather forecasting, forecasting weather, monitoring oceanic and atmospheric conditions, Hydrography, charting the seas, ...
's Geophysical Fluid Dynamics Laboratory show that above , the greenhouse gases dominate the cooling.
* Ozone depleting chemicals are also often greenhouse gases. The increases in concentrations of these chemicals have produced 0.34 ± 0.03 W/m2 of radiative forcing, corresponding to about 14 percent of the total radiative forcing from increases in the concentrations of well-mixed greenhouse gases.fossil fuels
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geologica ...
.)
In 2019, NASA reported that there was no significant relation between size of the ozone hole and climate change.
Misconceptions
CFC weight
Since CFC molecules are heavier than air (nitrogen or oxygen), it is commonly believed that the CFC molecules cannot reach the stratosphere in significant amounts. However, atmospheric gases are not sorted by weight at these altitudes; the forces of wind can fully mix the gases in the atmosphere. Some of the heavier CFCs are not evenly distributed.
Percentage of human-made chlorine
 Another misconception is that natural sources of chlorine are several times larger than human-made ones. While this statement is true for tropospheric chlorine, that is irrelevant to ozone depletion, which is only affected by stratospheric chlorine. Chlorine from ocean spray is soluble and thus is washed by rainfall before it reaches the stratosphere. CFCs, in contrast, are insoluble and long-lived, allowing them to reach the stratosphere. In the lower atmosphere, there is much more chlorine from CFCs and related haloalkanes than there is in HCl from salt spray, and in the stratosphere halocarbons are dominant. Only methyl chloride, which is one of these halocarbons, has a mainly natural source, and it is responsible for about 20 percent of the chlorine in the stratosphere; the remaining 80 percent comes from human-made sources.
Very violent volcanic eruptions can inject HCl into the stratosphere, but researchers have shown that the contribution is not significant compared to that from CFCs.
Another misconception is that natural sources of chlorine are several times larger than human-made ones. While this statement is true for tropospheric chlorine, that is irrelevant to ozone depletion, which is only affected by stratospheric chlorine. Chlorine from ocean spray is soluble and thus is washed by rainfall before it reaches the stratosphere. CFCs, in contrast, are insoluble and long-lived, allowing them to reach the stratosphere. In the lower atmosphere, there is much more chlorine from CFCs and related haloalkanes than there is in HCl from salt spray, and in the stratosphere halocarbons are dominant. Only methyl chloride, which is one of these halocarbons, has a mainly natural source, and it is responsible for about 20 percent of the chlorine in the stratosphere; the remaining 80 percent comes from human-made sources.
Very violent volcanic eruptions can inject HCl into the stratosphere, but researchers have shown that the contribution is not significant compared to that from CFCs.[ozone-depletion FAQ, Part II](_blank)
, section 4.4 A similar erroneous assertion is that soluble halogen compounds from the volcanic plume of Mount Erebus on Ross Island, Antarctica are a major contributor to the Antarctic ozone hole.hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
(HCl)) can reach the Antarctic stratosphere via high-latitude cyclones and then the polar vortex. Depending on the level of its volcanic activity, the additional annual HCl mass entering the stratosphere from Erebus varies from 1.0 to 14.3 kt.
First observation
G.M.B. Dobson mentioned that when springtime ozone levels in the Antarctic over Halley Bay were first measured in 1956, he was surprised to find that they were only about 320 DU, about 150 DU below typical spring Arctic levels of around 450 DU. What Dobson observed was not an ozone hole but in fact a typical annual maximum Antarctic ozone concentration: actual ozone hole values are in the 150–100 DU range. While Arctic ozone concentrations vary on a smooth annual cycle from around 300 to 450 DU, peaking in the northern hemisphere spring, Antarctic concentrations drop sharply in the southern hemisphere spring from highs of around 300 DU to much lower values. Peak values are not reached again until December.
Location of hole
Some people thought that the ozone hole should be above the sources of CFCs. However, CFCs are well mixed globally in the troposphere
The troposphere is the lowest layer of the atmosphere of Earth. It contains 80% of the total mass of the Atmosphere, planetary atmosphere and 99% of the total mass of water vapor and aerosols, and is where most weather phenomena occur. From the ...
and stratosphere
The stratosphere () is the second-lowest layer of the atmosphere of Earth, located above the troposphere and below the mesosphere. The stratosphere is composed of stratified temperature zones, with the warmer layers of air located higher ...
. The reason for occurrence of the ozone hole above Antarctica is not because there are more CFCs concentrated but because the low temperatures help form polar stratospheric clouds. In fact, there are findings of significant and localized "ozone holes" above other parts of the Earth, such as above Central Asia.
Awareness campaigns
Public misconceptions and misunderstandings of complex issues like ozone depletion are common. The limited scientific knowledge of the public led to confusion about global warming or the perception of global warming as a subset of the "ozone hole". In the beginning, classical green NGOs refrained from using CFC depletion for campaigning, as they assumed the topic was too complicated.global warming
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes ...
theory predicts that the stratosphere will cool.
* When the Antarctic ozone hole breaks up each year, the ozone-depleted air drifts into nearby regions. Decreases in the ozone level of up to 10 percent have been reported in New Zealand in the month following the breakup of the Antarctic ozone hole, with ultraviolet-B radiation intensities increasing by more than 15 percent since the 1970s.
World Ozone Day
In 1994, the United Nations General Assembly
The United Nations General Assembly (UNGA or GA; , AGNU or AG) is one of the six principal organs of the United Nations (UN), serving as its main deliberative, policymaking, and representative organ. Currently in its Seventy-ninth session of th ...
voted to designate September 16 as the International Day for the Preservation of the Ozone Layer, or "World Ozone Day". The designation commemorates the signing of the Montreal Protocol
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 ...
on that date in 1987.
See also
* Climate change in the Arctic
* Section 608
References
Sources
*
Further reading
* Andersen, S. O. and K. M. Sarma. (2002). ''Protecting the Ozone Layer: The United Nations History'', Earthscan Press. London, England.
* (Ambassador Benedick was the Chief U.S. Negotiator at the meetings that resulted in the Montreal Protocol.)
* Chasek, Pamela S., David L. Downie, and Janet Welsh Brown (2013). ''Global Environmental Politics'', 6th ed., Boulder, Colorado: Westview Press.
*
*
* Haas, P. (1992)
Banning chlorofluorocarbons: Epistemic community efforts to protect stratospheric ozone
International Organization, 46(1), 187–224.
* Parson, Edward (2004). ''Protecting the Ozone Layer: Science and Strategy''. Oxford, England: Oxford University Press.
External links
*
NOAA/ESRL Ozone Depleting Gas Index
MACC stratospheric ozone service
delivers maps, datasets and validation reports about the past and current state of the ozone layer.
Green Cooling Initiative on alternative natural refrigerants cooling technologies
"Ozone Hole: How We Saved the Planet"
premiered April 10, 2019 PBS
"Whatever happened to the Ozone Hole?"
''Distillations'' Podcast Episode 230, April 17, 2018, Science History Institute
The Science History Institute is an institution that preserves and promotes understanding of the history of science. Located in Philadelphia, Pennsylvania, it includes a library, museum, archive, research center and conference center.
It was ...
{{Authority control
Anthropocene
Ozone depletion
Ozone depletion consists of two related events observed since the late 1970s: a lowered total amount of ozone in Earth, Earth's upper atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone layer) around Earth's polar ...
Air pollution
 Ozone depletion consists of two related events observed since the late 1970s: a lowered total amount of
Ozone depletion consists of two related events observed since the late 1970s: a lowered total amount of  The ozone hole is usually measured by reduction in the total ''column ozone'' above a point on the Earth's surface. This is normally expressed in Dobson units; abbreviated as "DU". The most prominent decrease in ozone has been in the lower stratosphere. Marked decreases in column ozone in the
The ozone hole is usually measured by reduction in the total ''column ozone'' above a point on the Earth's surface. This is normally expressed in Dobson units; abbreviated as "DU". The most prominent decrease in ozone has been in the lower stratosphere. Marked decreases in column ozone in the  The Antarctic ozone hole is an area of the Antarctic stratosphere in which the recent ozone levels have dropped to as low as 33 percent of their pre-1975 values. The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container. Within this polar vortex, over 50 percent of the lower stratospheric ozone is destroyed during the Antarctic spring.
As explained above, the primary cause of ozone depletion is the presence of chlorine-containing source gases (primarily CFCs and related halocarbons). In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is substantially enhanced in the presence of polar stratospheric clouds (PSCs).
These polar stratospheric clouds form during winter, in the extreme cold. Polar winters are dark, consisting of three months without solar radiation (sunlight). The lack of sunlight contributes to a decrease in temperature and the polar vortex traps and chills the air. Temperatures are around or below −80 °C. These low temperatures form cloud particles. There are three types of PSC clouds—nitric acid trihydrate clouds, slowly cooling water-ice clouds, and rapid cooling water-ice (nacreous) clouds—provide surfaces for chemical reactions whose products will, in the spring lead to ozone destruction.
The photochemical processes involved are complex but well understood. The key observation is that, ordinarily, most of the chlorine in the stratosphere resides in "reservoir" compounds, primarily chlorine nitrate () as well as stable end products such as HCl. The formation of end products essentially removes Cl from the ozone depletion process. Reservoir compounds sequester Cl, which can later be made available via absorption of light at wavelengths shorter than 400 nm. During the Antarctic winter and spring, reactions on the surface of the polar stratospheric cloud particles convert these "reservoir" compounds into reactive free radicals (Cl and ClO). Denitrification is the process by which the clouds remove from the stratosphere by converting it to nitric acid in PSC particles, which then are lost by sedimentation. This prevents newly formed ClO from being converted back into .
The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring. During winter, even though PSCs are at their most abundant, there is no light over the pole to drive chemical reactions. During the spring, however, sunlight returns and provides energy to drive photochemical reactions and melt the polar stratospheric clouds, releasing considerable ClO, which drives the hole mechanism. Further warming temperatures near the end of spring break up the vortex around mid-December. As warm, ozone and -rich air flows in from lower latitudes, the PSCs are destroyed, the enhanced ozone depletion process shuts down, and the ozone hole closes.
Most of the ozone that is destroyed is in the lower stratosphere, in contrast to the much smaller ozone depletion through homogeneous gas-phase reactions, which occurs primarily in the upper stratosphere.
The Antarctic ozone hole is an area of the Antarctic stratosphere in which the recent ozone levels have dropped to as low as 33 percent of their pre-1975 values. The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container. Within this polar vortex, over 50 percent of the lower stratospheric ozone is destroyed during the Antarctic spring.
As explained above, the primary cause of ozone depletion is the presence of chlorine-containing source gases (primarily CFCs and related halocarbons). In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is substantially enhanced in the presence of polar stratospheric clouds (PSCs).
These polar stratospheric clouds form during winter, in the extreme cold. Polar winters are dark, consisting of three months without solar radiation (sunlight). The lack of sunlight contributes to a decrease in temperature and the polar vortex traps and chills the air. Temperatures are around or below −80 °C. These low temperatures form cloud particles. There are three types of PSC clouds—nitric acid trihydrate clouds, slowly cooling water-ice clouds, and rapid cooling water-ice (nacreous) clouds—provide surfaces for chemical reactions whose products will, in the spring lead to ozone destruction.
The photochemical processes involved are complex but well understood. The key observation is that, ordinarily, most of the chlorine in the stratosphere resides in "reservoir" compounds, primarily chlorine nitrate () as well as stable end products such as HCl. The formation of end products essentially removes Cl from the ozone depletion process. Reservoir compounds sequester Cl, which can later be made available via absorption of light at wavelengths shorter than 400 nm. During the Antarctic winter and spring, reactions on the surface of the polar stratospheric cloud particles convert these "reservoir" compounds into reactive free radicals (Cl and ClO). Denitrification is the process by which the clouds remove from the stratosphere by converting it to nitric acid in PSC particles, which then are lost by sedimentation. This prevents newly formed ClO from being converted back into .
The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring. During winter, even though PSCs are at their most abundant, there is no light over the pole to drive chemical reactions. During the spring, however, sunlight returns and provides energy to drive photochemical reactions and melt the polar stratospheric clouds, releasing considerable ClO, which drives the hole mechanism. Further warming temperatures near the end of spring break up the vortex around mid-December. As warm, ozone and -rich air flows in from lower latitudes, the PSCs are destroyed, the enhanced ozone depletion process shuts down, and the ozone hole closes.
Most of the ozone that is destroyed is in the lower stratosphere, in contrast to the much smaller ozone depletion through homogeneous gas-phase reactions, which occurs primarily in the upper stratosphere.
 The full extent of the damage that CFCs have caused to the ozone layer is not known and will not be known for decades; however, marked decreases in column ozone have already been observed. The Montreal and Vienna conventions were installed long before a scientific consensus was established or important uncertainties in the science field were being resolved. The ozone case was understood comparably well by lay persons as e.g. ''Ozone shield'' or ''ozone hole'' were useful "easy-to-understand bridging metaphors". Americans voluntarily switched away from aerosol sprays, resulting in a 50 percent sales loss even before legislation was enforced.
After a 1976 report by the
The full extent of the damage that CFCs have caused to the ozone layer is not known and will not be known for decades; however, marked decreases in column ozone have already been observed. The Montreal and Vienna conventions were installed long before a scientific consensus was established or important uncertainties in the science field were being resolved. The ozone case was understood comparably well by lay persons as e.g. ''Ozone shield'' or ''ozone hole'' were useful "easy-to-understand bridging metaphors". Americans voluntarily switched away from aerosol sprays, resulting in a 50 percent sales loss even before legislation was enforced.
After a 1976 report by the  Since the adoption and strengthening of the
Since the adoption and strengthening of the  Another misconception is that natural sources of chlorine are several times larger than human-made ones. While this statement is true for tropospheric chlorine, that is irrelevant to ozone depletion, which is only affected by stratospheric chlorine. Chlorine from ocean spray is soluble and thus is washed by rainfall before it reaches the stratosphere. CFCs, in contrast, are insoluble and long-lived, allowing them to reach the stratosphere. In the lower atmosphere, there is much more chlorine from CFCs and related haloalkanes than there is in HCl from salt spray, and in the stratosphere halocarbons are dominant. Only methyl chloride, which is one of these halocarbons, has a mainly natural source, and it is responsible for about 20 percent of the chlorine in the stratosphere; the remaining 80 percent comes from human-made sources.
Very violent volcanic eruptions can inject HCl into the stratosphere, but researchers have shown that the contribution is not significant compared to that from CFCs.ozone-depletion FAQ, Part II
Another misconception is that natural sources of chlorine are several times larger than human-made ones. While this statement is true for tropospheric chlorine, that is irrelevant to ozone depletion, which is only affected by stratospheric chlorine. Chlorine from ocean spray is soluble and thus is washed by rainfall before it reaches the stratosphere. CFCs, in contrast, are insoluble and long-lived, allowing them to reach the stratosphere. In the lower atmosphere, there is much more chlorine from CFCs and related haloalkanes than there is in HCl from salt spray, and in the stratosphere halocarbons are dominant. Only methyl chloride, which is one of these halocarbons, has a mainly natural source, and it is responsible for about 20 percent of the chlorine in the stratosphere; the remaining 80 percent comes from human-made sources.
Very violent volcanic eruptions can inject HCl into the stratosphere, but researchers have shown that the contribution is not significant compared to that from CFCs.ozone-depletion FAQ, Part II