Oxidizing on:
[Wikipedia]
[Google]
[Amazon]
 Redox (reduction–oxidation, , ) is a type of
Redox (reduction–oxidation, , ) is a type of

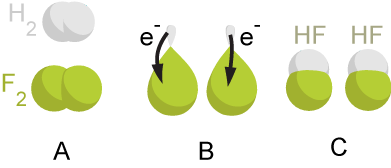 In the reaction between
In the reaction between
 In this type of reaction, a metal atom in a compound (or in a solution) is replaced by an atom of another metal. For example,
In this type of reaction, a metal atom in a compound (or in a solution) is replaced by an atom of another metal. For example,
 * The term
* The term
 Many important
Many important
 Minerals are generally oxidized derivatives of metals. Iron is mined as its
Minerals are generally oxidized derivatives of metals. Iron is mined as its
Chemical Equation Balancer
– An open-source chemical equation balancer that handles redox reactions.
Online redox reaction equation balancer, balances equations of any half-cell and full reactions
{{Authority control Soil chemistry Chemical reactions Articles containing video clips Redox Reaction mechanisms
 Redox (reduction–oxidation, , ) is a type of
Redox (reduction–oxidation, , ) is a type of chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
in which the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. ...
s of substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (locomotion), the surface over which an organism lo ...
change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
There are two classes of redox reactions:
* ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials.
* ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ...
, C=C (and other) bonds are reduced by transfer of hydrogen atoms.
Terminology
"Redox" is a combination of the words "reduction" and "oxidation". The term "redox" was first used in 1928. The processes of oxidation and reduction occur simultaneously and cannot occur independently. In redox processes, the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant or ''reducing agent'' loses electrons and is oxidized, and the oxidant or ''oxidizing agent'' gains electrons and is reduced. The pair of an oxidizing and reducing agent that is involved in a particular reaction is called a ''redox pair''. A ''redox couple'' is a reducing species and its corresponding oxidizing form, e.g., / .The oxidation alone and the reduction alone are each called a '' half-reaction'' because two half-reactions always occur together to form a whole reaction.Oxidants
''Oxidation'' originally implied a reaction with oxygen to form an oxide. Later, the term was expanded to encompass oxygen-like substances that accomplished parallel chemical reactions. Ultimately, the meaning was generalized to include all processes involving the loss of electrons. Substances that have the ability to ''oxidize'' other substances (cause them to lose electrons) are said to be ''oxidative'' or ''oxidizing'', and are known as oxidizing agents, oxidants, or oxidizers. The oxidant (oxidizing agent) removes electrons from another substance, and is thus itself reduced. And, because it "accepts" electrons, the oxidizing agent is also called anelectron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mist ...
. Oxidants are usually chemical substances with elements in high oxidation states (e.g., , , , , ), or else highly electronegative elements (e.g. O2, F2, Cl2, Br2, I2) that can gain extra electrons by oxidizing another substance.
Oxidizers are oxidants, but the term is mainly reserved for sources of oxygen, particularly in the context of explosions. Nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
is an oxidizer.
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
is the quintessential oxidizer.
Reducers
Substances that have the ability to ''reduce'' other substances (cause them to gain electrons) are said to be ''reductive'' or ''reducing'' and are known as reducing agents, reductants, or reducers. The reductant (reducing agent) transfers electrons to another substance and is thus itself oxidized. And, because it donates electrons, the reducing agent is also called an electron donor. Electron donors can also form charge transfer complexes with electron acceptors. The word ''reduction'' originally referred to the loss in weight upon heating a metallic ore such as a metal oxide to extract the metal. In other words, ore was "reduced" to metal. Antoine Lavoisier demonstrated that this loss of weight was due to the loss of oxygen as a gas. Later, scientists realized that the metal atom gains electrons in this process. The meaning of ''reduction'' then became generalized to include all processes involving a gain of electrons. Reducing equivalent refers to chemical species which transfer the equivalent of oneelectron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
in redox reactions. The term is common in biochemistry. A reducing equivalent can be an electron, a hydrogen atom, as a hydride ion.
Reductants in chemistry are very diverse. Electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
elemental metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
s, such as lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
, sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic t ...
, and aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately o ...
, are good reducing agents. These metals donate or ''give away'' electrons relatively readily. They transfer electrons.
''Hydride transfer reagents'', such as NaBH4 and LiAlH4, reduce by atom transfer: they transfer the equivalent of hydride or H−. These reagents widely used in the reduction of carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containin ...
compounds to alcohols. A related method of reduction involves the use of hydrogen gas (H2) as sources of H atoms.
Electronation and deelectronation
The electrochemist John Bockris proposed the words ''electronation'' and ''deelectronation'' to describe reduction and oxidation processes, respectively, when they occur atelectrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials ...
s. These words are analogous to protonation and deprotonation. They have not been widely adopted by chemists worldwide, although IUPAC has recognized the term electronation.
Rates, mechanisms, and energies
Redox reactions can occur slowly, as in the formation of rust, or rapidly, as in the case of burning fuel. Electron transfer reactions are generally fast, occurring within the time of mixing. The mechanisms of atom-transfer reactions are highly variable because many kinds of atoms can be transferred. Such reactions can also be quite complex, i.e. involve many steps. The mechanisms of electron-transfer reactions occur by two distinct pathways, inner sphere electron transfer and outer sphere electron transfer. Analysis of bond energies and ionization energies in water allow calculation of the thermodynamic aspects of redox reactions.Standard electrode potentials (reduction potentials)
Each half-reaction has a ''standardelectrode potential
In electrochemistry, electrode potential is the electromotive force of a galvanic cell built from a standard reference electrode and another electrode to be characterized. By convention, the reference electrode is the standard hydrogen electrod ...
'' (''E''), which is equal to the potential difference or voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge t ...
at equilibrium under standard conditions of an electrochemical cell in which the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction i ...
reaction is the half-reaction considered, and the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemoni ...
is a standard hydrogen electrode where hydrogen is oxidized:
: H2 → H+ + e−
The electrode potential of each half-reaction is also known as its ''reduction potential'' ''E'', or potential when the half-reaction takes place at a cathode. The reduction potential is a measure of the tendency of the oxidizing agent to be reduced. Its value is zero for H+ + e− → H2 by definition, positive for oxidizing agents stronger than H+ (e.g., +2.866 V for F2) and negative for oxidizing agents that are weaker than H+ (e.g., −0.763 V for Zn2+).
For a redox reaction that takes place in a cell, the potential difference is:
:''E'' = ''E'' – ''E''
However, the potential of the reaction at the anode is sometimes expressed as an ''oxidation potential'':
:''E'' = –''E''
The oxidation potential is a measure of the tendency of the reducing agent to be oxidized but does not represent the physical potential at an electrode. With this notation, the cell voltage equation is written with a plus sign
:''E'' = ''E'' + ''E''
Examples of redox reactions
 In the reaction between
In the reaction between hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
and fluorine, hydrogen is being oxidized and fluorine is being reduced:
:
This reaction is spontaneous and releases 542 kJ per 2 g of hydrogen because the H-F bond is much stronger than the F-F bond. This reaction can be analyzed as two half-reactions. The oxidation reaction converts hydrogen to protons:
:
The reduction reaction converts fluorine to the fluoride anion:
:
The half reactions are combined so that the electrons cancel:
:
The protons and fluoride combine to form hydrogen fluoride in a non-redox reaction:
:2 H+ + 2 F− → 2 HF
The overall reaction is:
:
Metal displacement
copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
is deposited when zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic t ...
metal is placed in a copper(II) sulfate solution:
:
In the above reaction, zinc metal displaces the copper(II) ion from copper sulfate solution and thus liberates free copper metal. The reaction is spontaneous and releases 213 kJ per 65 g of zinc.
The ionic equation for this reaction is:
:
As two half-reactions, it is seen that the zinc is oxidized:
:
And the copper is reduced:
:
Other examples
* The reduction of nitrate tonitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
in the presence of an acid ( denitrification):
::
* The combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combust ...
of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s, such as in an internal combustion engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal co ...
, produces water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
, carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, some partially oxidized forms such as carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
, and heat energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of hea ...
. Complete oxidation of materials containing carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes ...
produces carbon dioxide.
* The stepwise oxidation of a hydrocarbon by oxygen, in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, produces water and, successively: an alcohol, an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
or a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
, a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
, and then a peroxide.
Corrosion and rusting
 * The term
* The term corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
refers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion; it forms as a result of the oxidation of iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
metal. Common rust often refers to iron(III) oxide, formed in the following chemical reaction:
::
* The oxidation of iron(II) to iron(III) by hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3% ...
in the presence of an acid:
::
::
:Here the overall equation involves adding the reduction equation to twice the oxidation equation, so that the electrons cancel:
::
Disproportionation
Adisproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term ca ...
reaction is one in which a single substance is both oxidized and reduced. For example, thiosulfate ion with sulfur in oxidation state +2 can react in the presence of acid to form elemental sulfur (oxidation state 0) and sulfur dioxide
Sulfur dioxide ( IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic ...
(oxidation state +4).
:
Thus one sulfur atom is reduced from +2 to 0, while the other is oxidized from +2 to +4.
Redox reactions in industry
Cathodic protection
Cathodic protection (CP; ) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects the metal to be protected to a more easily corroded " sacri ...
is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects protected metal to a more easily corroded " sacrificial anode" to act as the anode. The sacrificial metal instead of the protected metal, then, corrodes. A common application of cathodic protection is in galvanized steel, in which a sacrificial coating of zinc on steel parts protects them from rust.
Oxidation is used in a wide variety of industries such as in the production of cleaning products and oxidizing ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
to produce nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
.
Redox reactions are the foundation of electrochemical cells, which can generate electrical energy or support electrosynthesis. Metal ores often contain metals in oxidized states such as oxides or sulfides, from which the pure metals are extracted by smelting
Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including silver, iron, copper, and other base metals. Smelting uses heat and a ...
at high temperature in the presence of a reducing agent. The process of electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the redox, reduction of cations of that metal by means of a direct current, direct electric cur ...
uses redox reactions to coat objects with a thin layer of a material, as in chrome-plated automotive parts, silver plating cutlery, galvanization
Galvanization or galvanizing ( also spelled galvanisation or galvanising) is the process of applying a protective zinc coating to steel or iron, to prevent rusting. The most common method is hot-dip galvanizing, in which the parts are submerged ...
and gold-plated jewelry
Jewellery (British English, UK) or jewelry (American English, U.S.) consists of decorative items worn for personal adornment, such as brooches, ring (jewellery), rings, necklaces, earrings, pendants, bracelets, and cufflinks. Jewellery may be at ...
.
Redox reactions in biology
Top:
Bottom: dehydroascorbic acid ( oxidized form of
ascorbic acid
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) a ...
( reduced form of Vitamin C
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) a ...
)Bottom: dehydroascorbic acid ( oxidized form of
Vitamin C
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) a ...
) Many important
Many important biological
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary ...
processes involve redox reactions. Before some of these processes can begin iron must be assimilated from the environment.
Cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
, for instance, is the oxidation of glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, usi ...
(C6H12O6) to CO2 and the reduction of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
to water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
. The summary equation for cell respiration is:
:
The process of cell respiration also depends heavily on the reduction of NAD+ to NADH and the reverse reaction (the oxidation of NADH to NAD+). Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
and cellular respiration are complementary, but photosynthesis is not the reverse of the redox reaction in cell respiration:
:
Biological energy is frequently stored and released by means of redox reactions. Photosynthesis involves the reduction of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
into sugars and the oxidation of water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
into molecular oxygen. The reverse reaction, respiration, oxidizes sugars to produce carbon dioxide and water. As intermediate steps, the reduced carbon compounds are used to reduce nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an ...
(NAD+) to NADH, which then contributes to the creation of a proton gradient, which drives the synthesis of adenosine triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms ...
(ATP) and is maintained by the reduction of oxygen.
In animal cells, mitochondria perform similar functions. See the ''Membrane potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charge ...
'' article.
Free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
reactions are redox reactions that occur as a part of homeostasis
In biology, homeostasis (British English, British also homoeostasis) Help:IPA/English, (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physics, physical, and chemistry, chemical conditions maintained by organism, living systems. Thi ...
and killing microorganisms, where an electron detaches from a molecule and then reattaches almost instantaneously. Free radicals are a part of redox molecules and can become harmful to the human body if they do not reattach to the redox molecule or an antioxidant. Unsatisfied free radicals can spur the mutation of cells they encounter and are, thus, causes of cancer.
The term ''redox state'' is often used to describe the balance of GSH/GSSG, NAD+/NADH and NADP+/NADPH in a biological system such as a cell or organ. The redox state is reflected in the balance of several sets of metabolites (e.g., lactate
Lactate may refer to:
* Lactation, the secretion of milk from the mammary glands
* Lactate, the conjugate base of lactic acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with ...
and pyruvate, beta-hydroxybutyrate, and acetoacetate
Acetoacetic acid (also acetoacetate and diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stab ...
), whose interconversion is dependent on these ratios. An abnormal redox state can develop in a variety of deleterious situations, such as hypoxia, shock, and sepsis
Sepsis, formerly known as septicemia (septicaemia in British English) or blood poisoning, is a life-threatening condition that arises when the body's response to infection causes injury to its own tissues and organs. This initial stage is foll ...
. Redox mechanism also control some cellular processes. Redox proteins and their genes must be co-located for redox regulation according to the CoRR hypothesis for the function of DNA in mitochondria and chloroplasts.
Redox cycling
Wide varieties of aromatic compounds are enzymatically reduced to form free radicals that contain one more electron than their parent compounds. In general, the electron donor is any of a wide variety of flavoenzymes and their coenzymes. Once formed, these anion free radicals reduce molecular oxygen to superoxide and regenerate the unchanged parent compound. The net reaction is the oxidation of the flavoenzyme's coenzymes and the reduction of molecular oxygen to form superoxide. This catalytic behavior has been described as afutile cycle
A futile cycle, also known as a substrate cycle, occurs when two metabolic pathways run simultaneously in opposite directions and have no overall effect other than to dissipate energy in the form of heat. The reason this cycle was called "futile" ...
or redox cycling.
Redox reactions in geology
 Minerals are generally oxidized derivatives of metals. Iron is mined as its
Minerals are generally oxidized derivatives of metals. Iron is mined as its magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With ...
(Fe3O4). Titanium is mined as its dioxide, usually in the form of rutile
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible wa ...
(TiO2). To obtain the corresponding metals, these oxides must be reduced, which is often achieved by heating these oxides with carbon or carbon monoxide as reducing agents. Blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being "forced" or supplied above atmospheric p ...
s are the reactors where iron oxides and coke (a form of carbon) are combined to produce molten iron.The main chemical reaction producing the molten iron is:
:
Redox reactions in soils
Electron transfer reactions are central to myriad processes and properties in soils, and electron "activity", quantified as Eh (platinum electrode potential (voltage) relative to the standard hydrogen electrode) or pe (analogous to pH as -log electron activity), is a master variable, along with pH, that controls and is governed by chemical reactions and biological processes. Early theoretical research with applications to flooded soils and paddy rice production was seminal for subsequent work on thermodynamic aspects of redox and plant root growth in soils. Later work built on this foundation, and expanded it for understanding redox reactions related to heavy metal oxidation state changes, pedogenesis and morphology, organic compound degradation and formation, free radical chemistry, wetland delineation, soil remediation, and various methodological approaches for characterizing the redox status of soils.Mnemonics
The key terms involved in redox can be confusing. For example, a reagent that is oxidized loses electrons; however, that reagent is referred to as the reducing agent. Likewise, a reagent that is reduced gains electrons and is referred to as the oxidizing agent. These mnemonics are commonly used by students to help memorise the terminology: * " OIL RIG" — oxidation is loss of electrons, reduction is gain of electrons * "LEO the lion says GER rr — loss of electrons is oxidation, gain of electrons is reduction * "LEORA says GEROA" — the loss of electrons is called oxidation (reducing agent); the gain of electrons is called reduction (oxidizing agent). * "RED CAT" and "AN OX", or "AnOx RedCat" ("an ox-red cat") — reduction occurs at the cathode and the anode is for oxidation * "RED CAT gains what AN OX loses" – reduction at the cathode gains (electrons) what anode oxidation loses (electrons) * "PANIC" – Positive Anode and Negative is Cathode. This applies toelectrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would not otherwise occur. The external energy source is a voltage applied between the cell′s two elect ...
s which release stored electricity, and can be recharged with electricity. PANIC does not apply to cells that can be recharged with redox materials. These galvanic or voltaic cells, such as fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
s, produce electricity from internal redox reactions. Here, the positive electrode is the cathode and the negative is the anode.
See also
*Anaerobic respiration
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
In aerobic organisms undergoing ...
* Bessemer process
* Bioremediation
Bioremediation broadly refers to any process wherein a biological system (typically bacteria, microalgae, fungi, and plants), living or dead, is employed for removing environmental pollutants from air, water, soil, flue gasses, industrial effluent ...
* Calvin cycle
* Chemical equation
* Chemical looping combustion
* Citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and prote ...
* Electrochemical series
* Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outc ...
* Electrolysis
* Electron equivalent
* Electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couple ...
* Electrosynthesis
* Galvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus ...
* Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ...
* Membrane potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charge ...
* Microbial fuel cell
* Murburn concept
* Nucleophilic abstraction
* Organic redox reaction
* Oxidative addition and reductive elimination
* Oxidative phosphorylation
Oxidative phosphorylation (UK , US ) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine t ...
* Partial oxidation
* Pro-oxidant
* Redox gradient
* Redox potential
* Reducing agent
* Reducing atmosphere
* Reduction potential
* Thermic reaction
* Transmetalation
* Sulfur cycle
References
Further reading
* *External links
Chemical Equation Balancer
– An open-source chemical equation balancer that handles redox reactions.
Online redox reaction equation balancer, balances equations of any half-cell and full reactions
{{Authority control Soil chemistry Chemical reactions Articles containing video clips Redox Reaction mechanisms