Oseltamivir total synthesis on:
[Wikipedia]
[Google]
[Amazon]
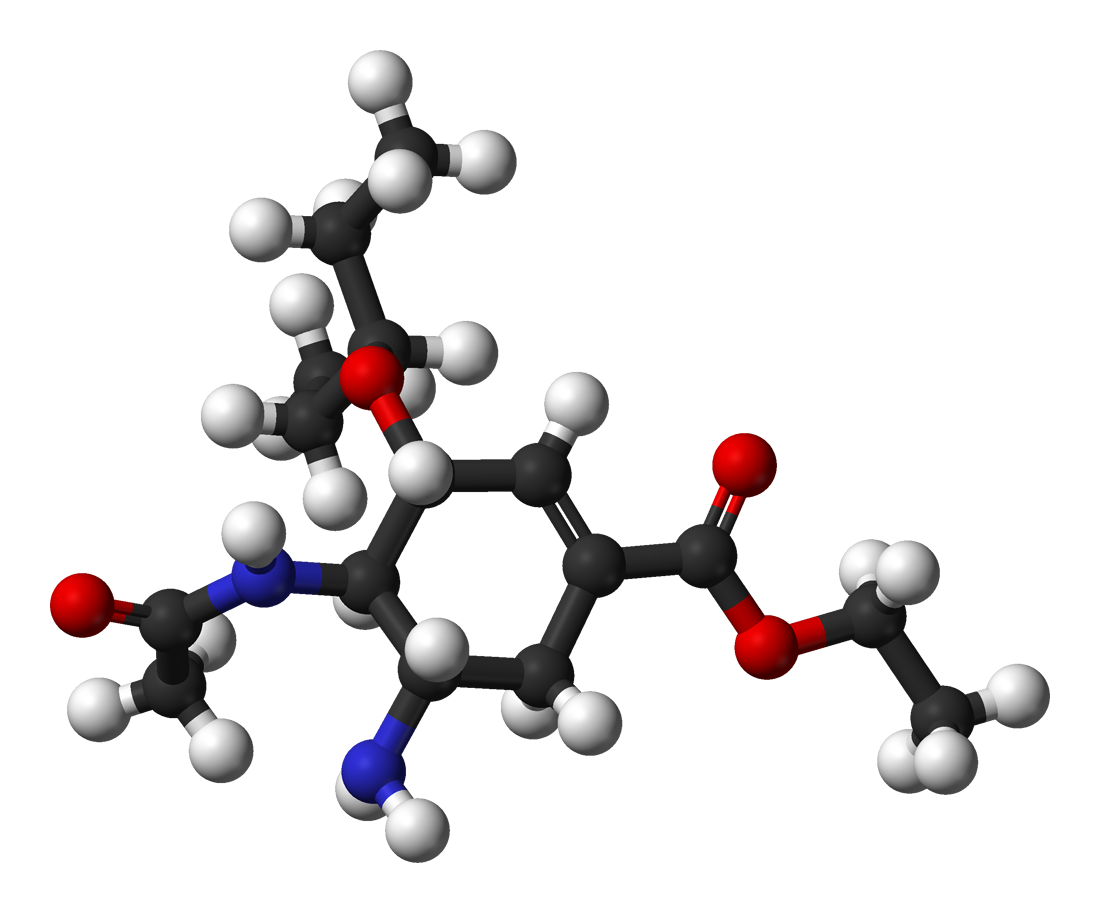 Oseltamivir total synthesis concerns the total synthesis of the antiinfluenza drug
Oseltamivir total synthesis concerns the total synthesis of the antiinfluenza drug


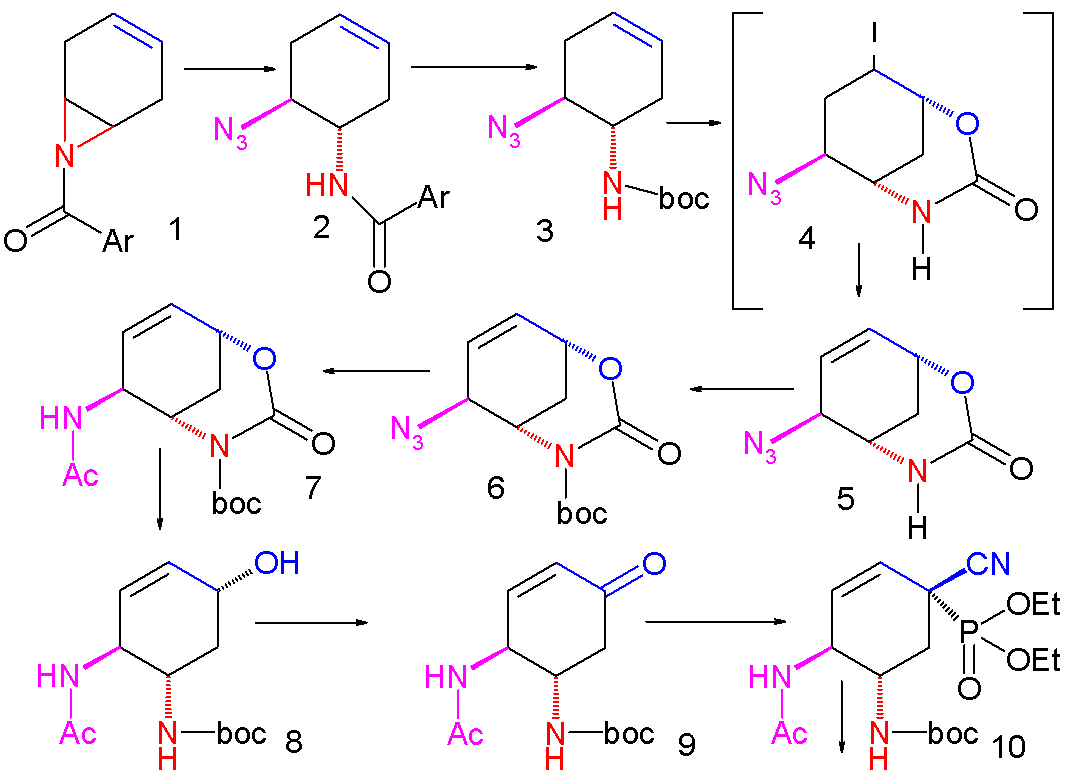
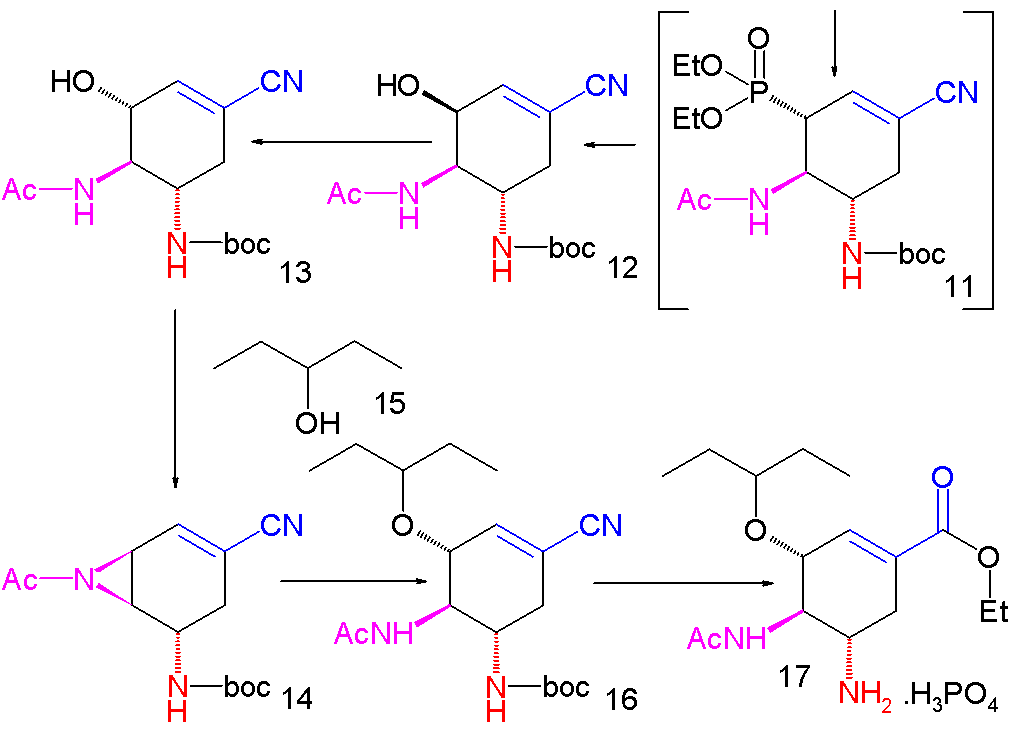 An improved method published in 2007 starts with the
An improved method published in 2007 starts with the

 Pyridine (1) is reduced with sodium borohydride in presence of
Pyridine (1) is reduced with sodium borohydride in presence of
 In 2008 the group of Barry M. Trost of
In 2008 the group of Barry M. Trost of
 In the first one-pot operation, Hayashi et al. begins by using diphenylprolinol silyl ether (4) as an
In the first one-pot operation, Hayashi et al. begins by using diphenylprolinol silyl ether (4) as an  In the second one-pot operation,
In the second one-pot operation,  The final one-pot operation begins with a
The final one-pot operation begins with a
Oseltamivir Total Syntheses @ SynArchive.com
{{DEFAULTSORT:Oseltamivir Total Synthesis Total synthesis Neuraminidase inhibitors
 Oseltamivir total synthesis concerns the total synthesis of the antiinfluenza drug
Oseltamivir total synthesis concerns the total synthesis of the antiinfluenza drug oseltamivir
Oseltamivir, sold under the brand name Tamiflu, is an antiviral medication used to treat and prevent influenza A and influenza B, viruses that cause the flu. Many medical organizations recommend it in people who have complications or are at hig ...
marketed by Hoffmann-La Roche
F. Hoffmann-La Roche AG, commonly known as Roche, is a Swiss multinational healthcare company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, Roche Holding AG, has shares listed on the SIX ...
under the trade name
A trade name, trading name, or business name, is a pseudonym used by companies that do not operate under their registered company name. The term for this type of alternative name is a "fictitious" business name. Registering the fictitious name w ...
''Tamiflu''. Its commercial production starts from the biomolecule
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large ...
shikimic acid harvested from Chinese star anise
''Illicium verum'' is a medium-sized evergreen tree native to northeast Vietnam and southwest China. A spice commonly called star anise, staranise, star anise seed, star aniseed, star of anise, Chinese star anise, or badian that closely resembl ...
and from recombinant E. coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
. Control of stereochemistry is important: the molecule has three stereocenters and the sought-after isomer is only 1 of 8 stereoisomers.
Commercial production
The current production method is based on the first scalable synthesis developed by Gilead Sciences starting from naturally occurringquinic acid
Quinic acid is a cyclitol, a cyclic polyol, and a cyclohexanecarboxylic acid. It is a colorless solid that can be extracted from plant sources. Quinic acid is implicated in the perceived acidity of coffee.
Occurrence and preparation
The compound ...
or shikimic acid. Due to lower yields and the extra steps required (because of the additional dehydration), the quinic acid route was dropped in favour of the one based on shikimic acid, which received further improvements by Hoffmann-La Roche
F. Hoffmann-La Roche AG, commonly known as Roche, is a Swiss multinational healthcare company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, Roche Holding AG, has shares listed on the SIX ...
.
The current industrial synthesis is summarised below:

Karpf / Trussardi synthesis
The current production method includes two reaction steps with potentially hazardousazide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applic ...
s. A reported azide-free Roche synthesis of tamiflu is summarised graphically below:
The synthesis commences from naturally available (−)- shikimic acid. The 3,4-pentylidene acetal mesylate
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the group ...
is prepared in three steps: esterification with ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
and thionyl chloride; ketal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments no ...
ization with ''p''-toluenesulfonic acid and 3-pentanone; and mesylation with triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
and methanesulfonyl chloride
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula . Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group –, it is frequently abbreviated MsCl in reaction schemes or equations. I ...
. Reductive opening of the ketal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments no ...
under modified Hunter conditions in dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
yields an inseparable mixture of isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
ic mesylates. The corresponding epoxide is formed under basic conditions with potassium bicarbonate. Using the inexpensive Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
magnesium bromide diethyl etherate (commonly prepared fresh by the addition of magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
turnings to 1,2-dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula . Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a ...
in benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
:diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
), the epoxide is opened with allyl amine to yield the corresponding 1,2-amino alcohol. The water-immiscible solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s methyl tert-butyl ether
Methyl ''tertiary''-butyl ether (MTBE), also known as methyl tert-butyl ether and ''tert''-butyl methyl ether, is an organic compound with a structural formula (CH3)3COCH3. MTBE is a volatile, flammable, and colorless liquid that is sparingly sol ...
and acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
are used to simplify the workup procedure, which involved stirring with 1 M aqueous ammonium sulfate. Reduction on palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
, promoted by ethanolamine
Ethanolamine (2-aminoethanol, monoethanolamine, ETA, or MEA) is an organic chemical compound with the formula or . The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid wit ...
, followed by acidic workup yielded the deprotected 1,2-aminoalcohol. The aminoalcohol was converted directly to the corresponding allyl-diamine in an interesting cascade sequence that commences with the unselective imination of benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
with azeotropic
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This ...
water removal in methyl tert-butyl ether. Mesylation, followed by removal of the solid byproduct triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
hydrochloride, results in an intermediate that was poised to undergo aziridination upon transimination with another equivalent of allylamine. With the librated methanesulfonic acid
Methanesulfonic acid (MsOH) or methanesulphonic acid (in British English) is an organosulfuric, colorless liquid with the chemical formula and structure . It is the simplest of the alkylsulfonic acids (). Salts and esters of methanesulfonic aci ...
, the
aziridine
Aziridine is an organic compound consisting of the three-membered heterocycle . It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its derivati ...
opens cleanly to yield a diamine that immediately undergoes a second transimination. Acidic hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
then removed the imine. Selective acylation with acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a col ...
(under buffered conditions, the 5-amino group is protonated
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, i ...
owing to a considerable difference in p''K''a, 4.2 vs 7.9, preventing acetylation
:
In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply '' acetates''. Deacetylation is the oppo ...
) yields the desired ''N''-acetylated product in crystalline form upon extractive workup. Finally, deallylation as above, yielded the freebase
Freebase may refer to:
*Free base or freebase, the pure basic form of an amine, as opposed to its salt form
*Freebase (database), a former online database service
*Freebase (mixtape), ''Freebase'' (mixtape), 2014 mixtape by 2 Chainz
*An original ...
of oseltamivir, which was converted to the desired oseltamivir phosphate by treatment with phosphoric acid. The final product is obtained in high purity (99.7%) and an overall yield of 17-22% from (−)-shikimic acid. It is noted that the synthesis avoids the use of potentially explosive azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applic ...
reagents and intermediates; however, the synthesis actually used by Roche uses azides. Roche has other routes to
oseltamivir that do not involve the use of (−)-shikimic acid as a chiral pool starting material, such as a Diels-Alder route involving furan and ethyl acrylate
Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is ...
or an isophthalic acid route, which involves catalytic hydrogenation and enzymatic desymmetrization.
Corey synthesis
In 2006 the group ofE.J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many ...
published a novel route bypassing shikimic acid starting from butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
and acrylic acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a ...
. The inventors chose not to patent
A patent is a type of intellectual property that gives its owner the legal right to exclude others from making, using, or selling an invention for a limited period of time in exchange for publishing an enabling disclosure of the invention."A p ...
this procedure which is described below.
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
1 reacts in an asymmetric Diels-Alder reaction with the esterification product of acrylic acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a ...
and 2,2,2-trifluoroethanol
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethy ...
2 catalysed by the CBS catalyst. The ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
3 is converted into an amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
in 4 by reaction with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
and the next step to lactam
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words ''lactone'' + ''amide''.
Nomenclature
Greek prefixes in alphabetical order indicate ring size:
* α-Lactam (3-atom rings)
* β-Lacta ...
5 is an iodolactamization
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words '' lactone'' + ''amide''.
Nomenclature
Greek prefixes in alphabetical order indicate ring size:
* α-Lactam (3-atom rings)
* β-Lac ...
with iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
initiated by trimethylsilyltriflate. The amide group is fitted with a BOC protecting group by reaction with Boc anhydride in 6 and the iodine substituent is removed in an elimination reaction with DBU
The decibel (symbol: dB) is a relative unit of measurement equal to one tenth of a bel (B). It expresses the ratio of two values of a Power, root-power, and field quantities, power or root-power quantity on a logarithmic scale. Two signals whose ...
to the alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
7. Bromine is introduced in 8 by an allylic bromination with NBS and the amide group is cleaved with ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
and caesium carbonate accompanied by elimination of bromide to the diene ethyl ester 9. The newly formed double bond is functionalized with ''N''-bromoacetamide 10 catalyzed with
tin(IV) bromide
Tin(IV) bromide is the chemical compound SnBr4. It is a colourless low melting solid.
SnBr4 can be prepared by reaction of the elements at normal temperatures:
:Sn + 2Br2 → SnBr4
In aqueous solution Sn(H2O)64+ is the principal ionic species ...
with complete control of stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
. In the next step the bromine atom in 11 is displaced by the nitrogen atom in the amide group with the strong base KHMDS
Potassium bis(trimethylsilyl)amide (commonly abbreviated as KHMDS, Potassium(K) HexaMethylDiSilazide) or potassium hexamethyldisilazane is the chemical compound with the formula ((CH3)3Si)2NK. It is a strong, non-nucleophilic base with an approxim ...
to the aziridine
Aziridine is an organic compound consisting of the three-membered heterocycle . It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its derivati ...
12 which in turn is opened by reaction with 3-pentanol 13 to the ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
14. In the final step the BOC group is removed with phosphoric acid and the oseltamivir phosphate 15 is formed.
Shibasaki synthesis
Also in 2006 the group of Masakatsu Shibasaki of the University of Tokyo published a synthesis again bypassing shikimic acid.
 An improved method published in 2007 starts with the
An improved method published in 2007 starts with the enantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
desymmetrization
Desymmetrization in stereochemistry is the modification of a molecule that results in the loss of one or more symmetry elements. A common application of this class of reactions involves the introduction of chirality.Willis, Michael C. "Enantiose ...
of aziridine
Aziridine is an organic compound consisting of the three-membered heterocycle . It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its derivati ...
1 with trimethylsilyl azide (TMSN3) and a chiral catalyst to the azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applic ...
2. The amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
group is protected as a BOC group with Boc anhydride and DMAP in 3 and iodolactamization
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words '' lactone'' + ''amide''.
Nomenclature
Greek prefixes in alphabetical order indicate ring size:
* α-Lactam (3-atom rings)
* β-Lac ...
with iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
and potassium carbonate first gives the unstable intermediate 4 and then stable cyclic carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally o ...
5 after elimination of hydrogen iodide
Hydrogen iodide () is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under sta ...
with DBU
The decibel (symbol: dB) is a relative unit of measurement equal to one tenth of a bel (B). It expresses the ratio of two values of a Power, root-power, and field quantities, power or root-power quantity on a logarithmic scale. Two signals whose ...
.
The amide group is reprotected as BOC 6 and the azide group converted to the amide 7 by reductive acylation with thioacetic acid
Thioacetic acid is an organosulfur compound with the molecular formula . It is the sulfur analogue of acetic acid (), as implied by the ''thio-'' prefix. It is a yellow liquid with a strong thiol-like odor. It is used in organic synthesis for the ...
and 2,6-lutidine. Caesium carbonate accomplishes the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of the carbamate group to the alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
8 which is subsequently oxidized to ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
9 with Dess-Martin periodinane. Cyanophosphorylation with diethyl phosphorocyanidate (DEPC) modifies the ketone group to the cyanophosphate 10 paving the way for an intramolecular allylic rearrangement An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution.
In reaction conditions that favor a SN1 reactio ...
to unstable β-allyl phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phospho ...
11 (toluene, sealed tube) which is hydrolyzed to alcohol 12 with ammonium chloride. This hydroxyl group has the wrong stereochemistry and is therefore inverted in a Mitsunobu reaction with p-nitrobenzoic acid followed by hydrolysis of the p-nitrobenzoate to 13.
A second Mitsunobu reaction then forms the aziridine
Aziridine is an organic compound consisting of the three-membered heterocycle . It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its derivati ...
14 available for ring-opening reaction with 3-pentanol
3-Pentanol is one of the eight isomers of amyl alcohol. It is found naturally and has a role as a pheromone.
See also
* 2-Pentanol
2-Pentanol (IUPAC name: pentan-2-ol; also called ''sec''-amyl alcohol) is an organic chemical compound. It is use ...
catalyzed by boron trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bondin ...
to ether 15. In the final step the BOC group is removed (HCl) and phosphoric acid added to objective 16.
Fukuyama synthesis
An approach published in 2007 like Corey's starts by anasymmetric Diels-Alder reaction
Asymmetric may refer to:
*Asymmetry in geometry, chemistry, and physics
Computing
* Asymmetric cryptography, in public-key cryptography
*Asymmetric digital subscriber line, Internet connectivity
* Asymmetric multiprocessing, in computer architect ...
this time with starting materials pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
and acrolein
Acrolein (systematic name: propenal) is the simplest unsaturated aldehyde. It is a colorless liquid with a piercing, acrid smell. The smell of burnt fat (as when cooking oil is heated to its smoke point) is caused by glycerol in the burning fa ...
.

 Pyridine (1) is reduced with sodium borohydride in presence of
Pyridine (1) is reduced with sodium borohydride in presence of benzyl chloroformate
Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl (Cbz or Z) group. In its pure form it is a water-sensitive oily ...
to the Cbz protected
Protection is any measure taken to guard a thing against damage caused by outside forces. Protection can be provided to physical objects, including organisms, to systems, and to intangible things like civil and political rights. Although th ...
dihydropyridine 2. The asymmetric Diels-Alder reaction with acrolein
Acrolein (systematic name: propenal) is the simplest unsaturated aldehyde. It is a colorless liquid with a piercing, acrid smell. The smell of burnt fat (as when cooking oil is heated to its smoke point) is caused by glycerol in the burning fa ...
3 is carried out with the McMillan catalyst to the aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
4 as the endo isomer
In organic chemistry, ''endo''–''exo'' isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. The prefix ''endo'' is reserved for the isomer with the substituent located closest ...
which is oxidized to the carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
5 with sodium chlorite
Sodium chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant.
Use
The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and pape ...
, monopotassium phosphate
Monopotassium phosphate (MKP) (also, potassium dihydrogenphosphate, KDP, or monobasic potassium phosphate) is the inorganic compound with the formula KH2PO4. Together with dipotassium phosphate (K2HPO4.(H2O)x) it is often used as a fertilizer, f ...
and 2-methyl-2-butene
2-Methyl-2-butene, 2m2b, 2-methylbut-2-ene, also beta-isoamylene is an alkene hydrocarbon with the molecular formula C5H10.
Used as a free radical scavenger in trichloromethane (chloroform) and dichloromethane (methylene chloride).
John Snow, th ...
. Addition of bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simila ...
gives halolactonization
Iodolactonization (or, more generally, halolactonization) is an organic reaction that forms a ring (the lactone) by the addition of an oxygen and iodine across a carbon-carbon double bond. It is an intramolecular variant of the halohydrin synthesi ...
product 6 and after replacement of the Cbz protective group by a BOC protective group in 7 (hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
in the presence of di-''tert''-butyl dicarbonate) a carbonyl group is introduced in intermediate 8 by catalytic ruthenium(IV) oxide
Ruthenium(IV) oxide is the inorganic compound with the formula Ru O2. This black solid is the most common oxide of ruthenium. It is widely used as an electrocatalyst for producing chlorine, chlorine oxides, and O2. Like many dioxides, RuO2 adop ...
and sacrificial catalyst
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, material ...
sodium periodate. Addition of ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
cleaves the ester group to form amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
9 the alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
group of which is mesylated to compound 10. In the next step iodobenzene diacetate is added, converting the amide in a Hofmann rearrangement to the allyl carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally o ...
12 after capturing the intermediate isocyanate with allyl alcohol 11. On addition of sodium ethoxide
Sodium ethoxide, also referred to as sodium ethylate, is the ionic, organic compound with the formula , or NaOEt (Et = ethane). It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. ...
in ethanol three reactions take place simultaneously: cleavage of the amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
to form new an ethyl ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
group, displacement of the mesyl group by newly formed BOC protected amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
to an aziridine
Aziridine is an organic compound consisting of the three-membered heterocycle . It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its derivati ...
group and an elimination reaction forming the alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
group in 13 with liberation of HBr. In the final two steps the aziridine ring is opened by 3-pentanol
3-Pentanol is one of the eight isomers of amyl alcohol. It is found naturally and has a role as a pheromone.
See also
* 2-Pentanol
2-Pentanol (IUPAC name: pentan-2-ol; also called ''sec''-amyl alcohol) is an organic chemical compound. It is use ...
14 and boron trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bondin ...
to aminoether 15 with the BOC group replaced by an acyl group and on removal of the other amine protecting group ( Pd/C, Ph3P, and 1,3-dimethylbarbituric acid in ethanol) and addition of phosphoric acid oseltamivir 16 is obtained.
Trost synthesis
Stanford University
Stanford University, officially Leland Stanford Junior University, is a private research university in Stanford, California. The campus occupies , among the largest in the United States, and enrolls over 17,000 students. Stanford is consider ...
published the shortest synthetic route to date.
Hayashi synthesis
In 2009, Hayashi et al. successfully produced an efficient, low cost synthetic route to prepare (-)-oseltamivir (1). Their goal was to design a procedure that would be suitable for large-scale production. Keeping cost, yield, and number of synthetic steps in mind, anenantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
total synthesis of (1) was accomplished through three one-pot operations. Hayashi et al.'s use of one-pot operations allowed them to perform several reactions steps in a single pot, which ultimately minimized the number of purification steps needed, waste, and saved time.
 In the first one-pot operation, Hayashi et al. begins by using diphenylprolinol silyl ether (4) as an
In the first one-pot operation, Hayashi et al. begins by using diphenylprolinol silyl ether (4) as an organocatalyst
In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an Organic compound, organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements fo ...
, along with alkoxyaldehyde (2) and nitroalkene (3) to perform an asymmetric Michael reaction
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
, affording an enantioselective Michael adduct. Upon addition of a diethyl vinylphosphate derivative (5) to the Michael adduct, a domino Michael reaction
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
and Horner-Wadsworth-Emmons reaction occurs due to the phosphonate group produced from (5) to give an ethyl cyclohexenecarboxylate derivative along with two unwanted by-products. To transform the undesired by-products into the desired ethyl cyclohexencarboxylate derivative, the mixture of the product and by-products was treated with Cs2CO3 in ethanol. This induced a retro-Michael reaction on one by-product and a retro-aldol reaction
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry.
Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two carb ...
accompanied with a Horner-Wadsworth-Emmons reaction for the other. Both by-products were successfully converted to the desired derivative. Finally, the addition of ''p''-toluenethiol with Cs2CO3 gives (6) in a 70% yield after being purified by column chromatography, with the desired isomer dominating.
 In the second one-pot operation,
In the second one-pot operation, trifluoroacetic acid
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with a ...
is employed first to deprotect the ''tert''-butyl ester of (6); any excess reagent was removed via evaporation. The carboxylic acid produced as a result of the deprotection was then converted to an acyl chloride by oxalyl chloride and a catalytic amount of DMF. Finally, addition of sodium azide, in the last reaction of the second one-pot operation, produce the acyl azide (7) without any purification needed.
 The final one-pot operation begins with a
The final one-pot operation begins with a Curtius Rearrangement
The Curtius rearrangement (or Curtius reaction or Curtius degradation), first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a va ...
of acyl azide (7) to produce an isocyanate functional group at room temperature. The isocyanate derivative then reacts with acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
to yield the desired acetylamino moiety found in (1). This domino Curtius rearrangement and amide formation occurs in the absence of heat, which is extremely beneficial for reducing any possible hazard. The nitro moiety of (7) is reduced to the desired amine observed in (1) with Zn/HCl. Due to the harsh conditions of the nitro reduction, ammonia was used to neutralize the reaction. Potassium carbonate was then added to give (1), via a retro-Michael reaction of the thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
. (1) was then purified by an acid/base extraction. The overall yield for the total synthesis of (-)-oseltamivir is 57%. Hayashi et al. use of inexpensive, non-hazardous reagents has allowed for an efficient, high yielding synthetic route that can allow for vast amount of novel derivatives to be produced in hopes of combatting against viruses resistant to (-)-oseltamivir.
References
External links
Oseltamivir Total Syntheses @ SynArchive.com
{{DEFAULTSORT:Oseltamivir Total Synthesis Total synthesis Neuraminidase inhibitors