organobismuth chemistry on:
[Wikipedia]
[Google]
[Amazon]
 Organobismuth chemistry is the
Organobismuth chemistry is the
 Triorganobismuth(III) compounds are monomeric with pyramidal structures reminiscent of organophosphorus(III) chemistry. The halides however adopt hypervalent structures. This trend is illustrated by the sheet-like structure adopted by methylbismuth dichloride.
Organobismuth heterocycles are based on Bi(III). The cyclic compound
Triorganobismuth(III) compounds are monomeric with pyramidal structures reminiscent of organophosphorus(III) chemistry. The halides however adopt hypervalent structures. This trend is illustrated by the sheet-like structure adopted by methylbismuth dichloride.
Organobismuth heterocycles are based on Bi(III). The cyclic compound
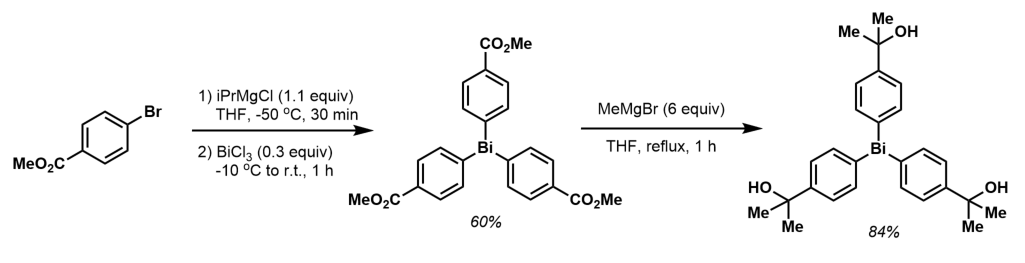 Asymmetric organobismuth compounds are those in which there is more than one type of organic group attached to the bismuth atom. The syntheses of these compounds proceed naturally and most conveniently from the organobismuth halides RBiX2 and R2BiX.
Asymmetric organobismuth compounds are those in which there is more than one type of organic group attached to the bismuth atom. The syntheses of these compounds proceed naturally and most conveniently from the organobismuth halides RBiX2 and R2BiX.
 Triphenylbismuth undergoes redistribution with its trihalide to give the mixed derivatives such as diphenylbismuth chloride (Ph2BiCl). Such reactions proceed more readily than for the lighter congeners.
Triarylbismuth(III) compounds may also be employed in C–N bond forming transformations with an appropriate metal co-catalyst. For instance, Barton and coworkers demonstrated that amines could be ''N''-arylated with a bismuth(III) reagent in the presence of copper(II) salt.
Triphenylbismuth undergoes redistribution with its trihalide to give the mixed derivatives such as diphenylbismuth chloride (Ph2BiCl). Such reactions proceed more readily than for the lighter congeners.
Triarylbismuth(III) compounds may also be employed in C–N bond forming transformations with an appropriate metal co-catalyst. For instance, Barton and coworkers demonstrated that amines could be ''N''-arylated with a bismuth(III) reagent in the presence of copper(II) salt.

 The above transformation proceeds through in an asynchronous concerted fashion from the ''O''-bound organobismuth(V) reagent after loss of an aryl group. A triarylbismuth(III) complex forms concomitantly. The regioselectivity of this transformation is guided by the directing ability of adjacent Lewis basic functionalities. It is important to note that in the above arylation, a full equivalent of the pentavalent bismuth compound is required for the arylation reaction, therefore leaving four ligands on bismuth inactive for further arylations. Catalytic manifolds of this chemistry are challenging due in part to the reoxidation of Bi(III) to Bi(V). For more examples of bismuth mediated arylations, see the cited review.
The above transformation proceeds through in an asynchronous concerted fashion from the ''O''-bound organobismuth(V) reagent after loss of an aryl group. A triarylbismuth(III) complex forms concomitantly. The regioselectivity of this transformation is guided by the directing ability of adjacent Lewis basic functionalities. It is important to note that in the above arylation, a full equivalent of the pentavalent bismuth compound is required for the arylation reaction, therefore leaving four ligands on bismuth inactive for further arylations. Catalytic manifolds of this chemistry are challenging due in part to the reoxidation of Bi(III) to Bi(V). For more examples of bismuth mediated arylations, see the cited review.
 Organobismuth chemistry is the
Organobismuth chemistry is the chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
of organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
containing a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
to bismuth
Bismuth is a chemical element with the Symbol (chemistry), symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental ...
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
. Applications are few. The main bismuth oxidation states are Bi(III) and Bi(V) as in all higher group 15 elements. The energy of a bond to carbon in this group decreases in the order P > As > Sb > Bi. The first reported use of bismuth in organic chemistry was in oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of alcohols by Challenger in 1934 (using Ph3Bi(OH)2). Knowledge about methylated species of bismuth in environmental and biological media is limited.
Discovery
Triethylbismuth, the first known organobismuth compound, is prepared in 1850 by Löwig and Schweizer fromiodoethane
Ethyl iodide (also iodoethane) is a colorless flammable chemical compound. It has the chemical formula C2H5I and is prepared by heating ethanol with iodine and phosphorus.''Merck Index of Chemicals and Drugs'', 9th ed., monograph 3753 On contact ...
and a potassium–bismuth alloy. As with most trialkylbismuth compounds, BiEt3 has an extremely pungent and unpleasant odor, and is spontaneously oxidized in air. The chemistry of these complexes first begin receiving significant attention when Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
s and organolithium compounds
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
become available.
OrganoBi(III) compounds
Properties and structure
bismole
Bismole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4 H4 BiH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with bismuth replacing the nitrogen atom of pyrrole. Th ...
, a structural analog
A structural analog (analogue in modern traditional English; Commonwealth English), also known as a chemical analog or simply an analog, is a compound having a structure similar to that of another compound, but differing from it in respect to a ce ...
of pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
, has not been isolated, but substituted bismoles are known. Bismabenzene
Bismabenzene () is the parent representative of a group of organobismuth compounds that are related to benzene with a carbon atom replaced by a bismuth atom. Bismabenzene itself has been synthesised but not isolated because it is too reactive, te ...
has been detected in the laboratory.
Synthesis
The most general and widely used methodology for the synthesis of homoleptic trialkyl- and triarylbismuth complexes is the reaction of BiX3 with organolithium or magnesium reagents generally of the form of a Grignard reagent: :BiCl3 + 3RMgX → R3Bi + 3MgXCl :BiCl3 + 3LiR → BiR3 + 3LiCl. The reaction of K3Bi with organic halides is the first method used to prepare triorganobismuth compounds: :K3Bi + 3RX → BiR3 + 3KX. This method is generally more difficult, not as clean, and produces a lower yield. In some cases, however, such as the synthesis of (Me3Si)3Bi, it is the only available method Triaryl bismuth(III) compounds are typically air-stable crystalline solids. The substituents on the triarylbismuth center can be modified. Asymmetric organobismuth compounds are those in which there is more than one type of organic group attached to the bismuth atom. The syntheses of these compounds proceed naturally and most conveniently from the organobismuth halides RBiX2 and R2BiX.
Asymmetric organobismuth compounds are those in which there is more than one type of organic group attached to the bismuth atom. The syntheses of these compounds proceed naturally and most conveniently from the organobismuth halides RBiX2 and R2BiX.
Reactions
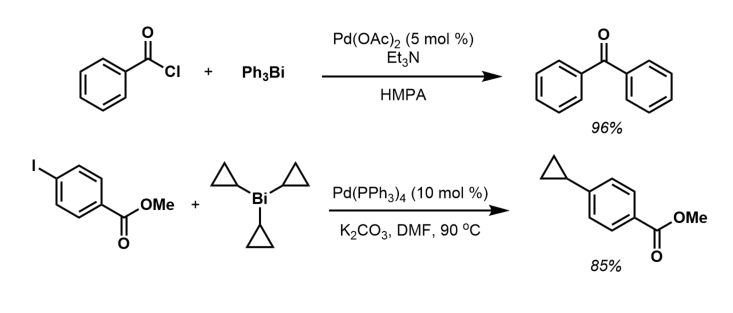
Triarylbismuth compounds have very limited use in organic synthesis. They react with acylchlorides under Pd(0) catalysis to form a variety of phenyl ketones. Tricyclopropylbismuth(III) reagents react with aryl halides and triflates under Pd(0) catalysis in a similar fashion to afford a variety of aryl and heteroaryl cyclopropanes. Triphenylbismuth undergoes redistribution with its trihalide to give the mixed derivatives such as diphenylbismuth chloride (Ph2BiCl). Such reactions proceed more readily than for the lighter congeners.
Triarylbismuth(III) compounds may also be employed in C–N bond forming transformations with an appropriate metal co-catalyst. For instance, Barton and coworkers demonstrated that amines could be ''N''-arylated with a bismuth(III) reagent in the presence of copper(II) salt.
Triphenylbismuth undergoes redistribution with its trihalide to give the mixed derivatives such as diphenylbismuth chloride (Ph2BiCl). Such reactions proceed more readily than for the lighter congeners.
Triarylbismuth(III) compounds may also be employed in C–N bond forming transformations with an appropriate metal co-catalyst. For instance, Barton and coworkers demonstrated that amines could be ''N''-arylated with a bismuth(III) reagent in the presence of copper(II) salt.

OrganoBi(V) compounds
Structure
Triarylorganobismuth complexes are easily oxidized to bismuth(V) complexes by treatment with chlorine or bromine, giving Ar3BiX2 (X = Cl, Br). Reactions with iodine result in elimination to give trivalent Ar3−xBiIx , while reactions with fluorine are too vigorous. The nature of the aryl ligands is important in determining whether the structure of those complexes is trigonal bipyramidal or square planar, which is also related to their color. Organobismuth(V) compounds of the type Ar5Bi adopt square pyramidal structures. The pentaphenyl compound is deeply colored andthermochromic
Thermochromism is the property of substances to change color due to a change in temperature. A mood ring is an excellent example of this phenomenon, but thermochromism also has more practical uses, such as baby bottles which change to a differen ...
, possibly because of an equilibrium between square pyramidal and trigonal bipyramidal structures.
Synthesis
Organobismuth(V) complexes may be accessed directly from organobismuth(III) through oxidative addition to a halogen then displacement of the newly formed bismuth-halogen bond for a bismuth-carbon bond with an alkyl or aryl lithium or Grignard reagent. Bi(V) compounds can be accessed through Bi(III) compounds for example: : Me3Bi + SO2Cl2 → Me3BiCl2 + SO2 : Me3BiCl2 + 2 MeLi → Me5Bi + 2 LiCl Bi(V) easily forms anonium ion
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen (group 15 of the periodic table), chalcogen (group 16), or halogen (group 17). The oldest-known onium ion, and the namesake f ...
for example by protonation with ''p''-toluenesulfonic acid:
: Ph5Bi + HO3SAr → Ph4Bi+ 3SAr−
Pentaphenylbismuth forms an ate complex
In chemistry, an ate complex is a salt formed by the reaction of a Lewis acid with a Lewis base whereby the central atom (from the Lewis acid) increases its valence and gains a negative formal charge. (In this definition, the meaning of valen ...
upon treatment with phenyl lithium
Phenyllithium or lithobenzene is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic synthe ...
:
: Ph5Bi + PhLi → Li+ h6Bi−
The thermal stability of R5M compounds decrease in the order As > Sb > Bi. The aryl compounds are more stable than alkyl compounds. Me5Bi decomposes explosively at 20°C.
Reactions
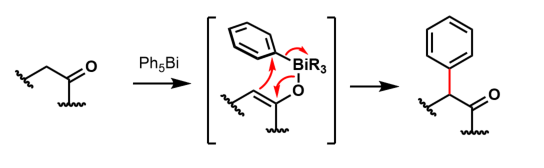
Compared to the lighter congeners, Bi(V) compounds are oxidizing. Reactions of Ar3BiX2 with organolithium reagents are common for producing Ar5Bi complexes. Unstable, purple Ph5Bi is the first of these to be synthesized. Organobismuth(V) reagents are useful for a wide variety of organic transformations including transfer reactions, oxidation of primary, secondary, benzylic, and allylic alcohols. These reagents also cleave glycols, and under the appropriate conditions function as aryl group transfer reagents. The compounds Ph3Bi(OOtBu)2, Ph3BiCO3 and (Ph3BiCl)2O have been investigated for the oxidation ofoximes
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted ...
, thiols
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
, phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are c ...
, and phosphines
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting f ...
. Compounds such as Ph5Bi and Ph3BiCl2 have been used in the arylation
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
of arene compounds and 1,3-dicarbonyl compounds:
:
References
{{ChemicalBondsToCarbon Organobismuth compounds