O-GlcNAcase on:
[Wikipedia]
[Google]
[Amazon]
Protein ''O''-GlcNAcase (, OGA, glycoside hydrolase ''O''-GlcNAcase, ''O''-GlcNAcase, BtGH84, ''O''-GlcNAc hydrolase) is an
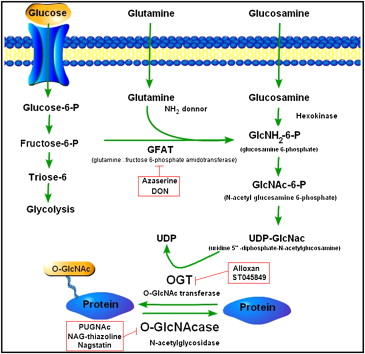 ''O''-GlcNAcylation is a form of
''O''-GlcNAcylation is a form of
 OGA catalyzes ''O''-GlcNAc hydrolysis via an
OGA catalyzes ''O''-GlcNAc hydrolysis via an
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
with systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature.
A semisystematic name or semitrivial ...
(protein)-3-''O''-(''N''-acetyl-D-glucosaminyl)-L-serine/threonine ''N''-acetylglucosaminyl hydrolase. OGA is encoded by the ''OGA'' gene. This enzyme catalyses the removal of the ''O''-GlcNAc post-translational modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosome ...
in the following chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
:
# rotein3-''O''-(''N''-acetyl-β-D-glucosaminyl)-L-serine + H2O roteinL-serine + ''N''-acetyl-D-glucosamine
# rotein3-''O''-(''N''-acetyl-β-D-glucosaminyl)-L-threonine + H2O roteinL-threonine + ''N''-acetyl-D-glucosamine
Nomenclature
Other names include: * Nuclear cytoplasmic ''O''-GlcNAcase and acetyltransferaseIsoforms
The human OGA gene is capable of producing two different transcripts, each capable of encoding a different OGA isoform. The long isoform L-OGA, a bifunctional enzyme that possess aglycoside hydrolase
Glycoside hydrolases (also called glycosidases or glycosyl hydrolases) catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes with roles in nature including degradation of biomass such as cellulose (cel ...
activity and a pseudo histone-acetyl transferase domain, primarily resides in the cytoplasm and the nucleus. The short isoform S-OGA, which only exhibit the glycoside hydrolase domain, was initially described as residing within the nucleus. However, more recent work showed that S-OGA is located in mitochondria and regulates reactive oxygen production in this organelle. Another isoform, resulting from proteolytic cleavage of L-OGA, has also been described. All three isoforms exhibit glycoside hydrolase activity.
Homologs
Protein ''O''-GlcNAcases belong to glycoside hydrolase family 84 of the carbohydrate active enzyme classification. Homologs exist in other species as ''O''-GlcNAcase is conserved in higher eukaryotic species. In a pairwise alignment, humans share 55% homology with ''Drosophila
''Drosophila'' () is a genus of flies, belonging to the family Drosophilidae, whose members are often called "small fruit flies" or (less frequently) pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species ...
'' and 43% with ''C. elegans
''Caenorhabditis elegans'' () is a free-living transparent nematode about 1 mm in length that lives in temperate soil environments. It is the type species of its genus. The name is a blend of the Greek ''caeno-'' (recent), ''rhabditis'' (r ...
''. ''Drosophila'' and ''C. elegans'' share 43% homology. Among mammals, the OGA sequence is even more highly conserved. The mouse and the human have 97.8% homology. However, OGA does not share significant homology with other proteins. However, short stretches of about 200 amino acids in OGA have homology with some proteins such as hyaluronidase, a putative acetyltransferase, eukaryotic translation elongation factor-1γ, and the 11-1 polypeptide.
Reaction
Protein ''O''-GlcNAcylation
 ''O''-GlcNAcylation is a form of
''O''-GlcNAcylation is a form of glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not al ...
, the site-specific enzymatic addition of saccharides to proteins and lipids. This form of glycosylation is with ''O''-linked β-''N''-acetylglucosamine or β-''O''-linked 2-acetamido-2-deoxy-D-glycopyranose (''O''-GlcNAc). In this form, a single sugar (β-''N''-acetylglucosamine) is added to serine and threonine residues of nuclear or cytoplasmic proteins. Two conserved enzymes control this glycosylation of serine and threonine: ''O''-GlcNAc transferase (OGT) and ''O''-GlcNAcase (OGA). While OGT catalyzes the addition of ''O''-GlcNAc to serine and threonine, OGA catalyzes the hydrolytic cleavage of ''O''-GlcNAc from post-transitionally modified proteins.
OGA is a member of the family of hexosaminidases
Hexosaminidase (, ''beta-acetylaminodeoxyhexosidase'', ''N-acetyl-beta-D-hexosaminidase'', ''N-acetyl-beta-hexosaminidase'', ''N-acetyl hexosaminidase'', ''beta-hexosaminidase'', ''beta-acetylhexosaminidinase'', ''beta-D-N-acetylhexosaminidase'', ...
. However, unlike lysosomal hexosaminidases, OGA activity is the highest at neutral pH (approximately 7) and it localizes mainly to the cytosol. OGA and OGT are synthesized from two conserved genes and are expressed throughout the human body with high levels in the brain and pancreas. The products of ''O''-GlcNAc and the process itself plays a role in embryonic development, brain activity, hormone production, and a myriad of other activities.
Over 600 proteins are targets for ''O''-GlcNAcylation. While the functional effects of ''O''-GlcNAc modification is not fully known, it is known that ''O''-GlcNAc modification impacts many cellular activities such as lipid/carbohydrate metabolism and hexosamine biosynthesis. Modified proteins may modulate various downstream signaling pathways by influencing transcription and proteomic activities.
Mechanism and inhibition
 OGA catalyzes ''O''-GlcNAc hydrolysis via an
OGA catalyzes ''O''-GlcNAc hydrolysis via an oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the ...
reaction intermediate. Stable compounds which mimic the reaction intermediate can act as selective enzyme inhibitors. Thiazoline
Thiazolines (or dihydrothiazoles) are a group of isomeric 5-membered heterocyclic compounds containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivatives are more common a ...
derivatives of GlcNAc can be used as a reaction intermediate. An example of this includes Thiamet-G as shown on the right. A second form of inhibition can occur from the mimicry of the transition state. The GlcNAcstatin family of inhibitors exploit this mechanism in order to inhibit OGA activity. For both types of inhibitors, OGA can be selected apart from the generic lysosomal hexosaminidases by elongating the C2 substituent in their chemical structure. This takes advantage of a deep pocket in OGA's active site that allow it to bind analogs of GlcNAc.
There is potential for regulation of ''O''-GlcNAcase for the treatment of Alzheimer's disease
Alzheimer's disease (AD) is a neurodegeneration, neurodegenerative disease that usually starts slowly and progressively worsens. It is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in short-term me ...
. When the tau protein
The tau proteins (abbreviated from tubulin associated unit) are a group of six highly soluble protein isoforms produced by alternative splicing from the gene ''MAPT'' (microtubule-associated protein tau). They have roles primarily in maintaining ...
in the brain is hyperphosphorylated, neurofibrillary tangles
Neurofibrillary tangles (NFTs) are aggregates of hyperphosphorylated tau protein that are most commonly known as a primary biomarker of Alzheimer's disease. Their presence is also found in numerous other diseases known as tauopathies. Little is k ...
form, which are a pathological hallmark for neurodegenerative diseases such as Alzheimer's disease. In order to treat this condition, OGA is targeted by inhibitors such as Thiamet-G in order to prevent ''O''-GlcNAc from being removed from tau, which assists in preventing tau from becoming phosphorylated.
Structure
X-ray structures are available for a range of ''O''-GlcNAcase proteins. The X-ray structure of human ''O''-GlcNAcase in complex with Thiamet-G identified the structural basis of enzyme inhibition.See also
* ''O''-GlcNAc * ''O''-GlcNAc transferase * ''O''-linked glycosylationReferences
Further reading
* * * * * * * * * * * *External links
* {{Portal bar, Biology, border=no EC 3.2.1