|
O-GlcNAcase
Protein ''O''-GlcNAcase (, OGA, glycoside hydrolase ''O''-GlcNAcase, ''O''-GlcNAcase, BtGH84, ''O''-GlcNAc hydrolase) is an enzyme with List of enzymes, systematic name (protein)-3-''O''-(''N''-acetyl-D-glucosaminyl)-L-serine/threonine ''N''-acetylglucosaminyl hydrolase. OGA is encoded by the ''OGA'' gene. This enzyme catalysis, catalyses the removal of the O-GlcNAc, ''O''-GlcNAc post-translational modification in the following chemical reaction: # [protein]-3-''O''-(''N''-acetyl-β-D-glucosaminyl)-L-serine + H2O [protein]-L-serine + ''N''-acetyl-D-glucosamine # [protein]-3-''O''-(''N''-acetyl-β-D-glucosaminyl)-L-threonine + H2O [protein]-L-threonine + ''N''-acetyl-D-glucosamine Nomenclature Other names include: * Nuclear cytoplasmic ''O''-GlcNAcase and acetyltransferase Isoforms The human OGA gene is capable of producing two different transcripts, each capable of encoding a different OGA isoform. The long isoform L-OGA, a bifunctional enzyme that possess a glycoside hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-GlcNAc
''O''-GlcNAc (short for ''O''-linked GlcNAc or ''O''-linked β-''N''-acetylglucosamine) is a reversible enzymatic post-translational modification that is found on serine and threonine residues of nucleocytoplasmic proteins. The modification is characterized by a β-glycosidic bond between the hydroxyl group of serine or threonine side chains and ''N''-acetylglucosamine (GlcNAc). ''O''-GlcNAc differs from other forms of protein glycosylation: (i) ''O''-GlcNAc is not elongated or modified to form more complex glycan structures, (ii) ''O''-GlcNAc is almost exclusively found on nuclear and cytoplasmic proteins rather than membrane proteins and secretory proteins, and (iii) ''O''-GlcNAc is a highly dynamic modification that turns over more rapidly than the proteins which it modifies. ''O''-GlcNAc is conserved across metazoans. Due to the dynamic nature of ''O''-GlcNAc and its presence on serine and threonine residues, ''O''-GlcNAcylation is similar to protein phosphorylation in some r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-linked Glycosylation
''O''-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. ''O''-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexosaminidases
Hexosaminidase (, ''beta-acetylaminodeoxyhexosidase'', ''N-acetyl-beta-D-hexosaminidase'', ''N-acetyl-beta-hexosaminidase'', ''N-acetyl hexosaminidase'', ''beta-hexosaminidase'', ''beta-acetylhexosaminidinase'', ''beta-D-N-acetylhexosaminidase'', ''beta-N-acetyl-D-hexosaminidase'', ''beta-N-acetylglucosaminidase'', ''hexosaminidase A'', ''N-acetylhexosaminidase'', ''beta-D-hexosaminidase'') is an enzyme involved in the hydrolysis of terminal N-acetyl-D-hexosamine residues in N-acetyl-β-D-hexosaminides. Elevated levels of hexosaminidase in blood and/or urine have been proposed as a biomarker of relapse in the treatment of alcoholism. Hereditary inability to form functional hexosaminidase enzymes are the cause of lipid storage disorders Tay-Sachs disease and Sandhoff disease. Isozymes and genes Lysosomal A, B, and S isozymes Functional lysosomal β-hexosaminidase enzymes are dimeric in structure. Three isozymes are produced through the combination of α and β subunits to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-GlcNAc Transferase
Protein ''O''-GlcNAc transferase also known as OGT or O-linked N-acetylglucosaminyltransferase is an enzyme () that in humans is encoded by the ''OGT'' gene. OGT catalyzes the addition of the ''O''-GlcNAc post-translational modification to proteins. Nomenclature Other names include: *''O''-GlcNAc transferase * OGTase *''O''-linked ''N''-acetylglucosaminyltransferase * Uridine diphospho-''N''-acetylglucosamine:polypeptide β-''N''-acetylglucosaminyltransferase Systematic name: UDP-''N''-α-acetyl--glucosamine: rotein3-''O''-''N''-acetyl-β--glucosaminyl transferase Function Glycosyltransferase OGT catalyzes the addition of a single ''N''-acetylglucosamine through an ''O''-glycosidic linkage to serine or threonine and an ''S''-glycosidic linkage to cysteine residues of nucleocytoplasmic proteins. Since both phosphorylation and ''O''-GlcNAcylation compete for similar serine or threonine residues, the two processes may compete for sites, or they may alter the substrate s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein O-GlcNAc Transferase
Protein ''O''-GlcNAc transferase also known as OGT or O-linked N-acetylglucosaminyltransferase is an enzyme () that in humans is encoded by the ''OGT'' gene. OGT catalyzes the addition of the ''O''-GlcNAc post-translational modification to proteins. Nomenclature Other names include: *''O''-GlcNAc transferase * OGTase *''O''-linked ''N''-acetylglucosaminyltransferase * Uridine diphospho-''N''-acetylglucosamine:polypeptide β-''N''-acetylglucosaminyltransferase Systematic name: UDP-''N''-α-acetyl--glucosamine: rotein3-''O''-''N''-acetyl-β--glucosaminyl transferase Function Glycosyltransferase OGT catalyzes the addition of a single ''N''-acetylglucosamine through an ''O''-glycosidic linkage to serine or threonine and an ''S''-glycosidic linkage to cysteine residues of nucleocytoplasmic proteins. Since both phosphorylation and ''O''-GlcNAcylation compete for similar serine or threonine residues, the two processes may compete for sites, or they may alter the substrate s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside Hydrolase
Glycoside hydrolases (also called glycosidases or glycosyl hydrolases) catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies (e.g., lysozyme), in pathogenesis mechanisms (e.g., viral neuraminidases) and in normal cellular function (e.g., trimming mannosidases involved in N-linked glycoprotein biosynthesis). Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds. Occurrence and importance Glycoside hydrolases are found in essentially all domains of life. In prokaryotes, they are found both as intracellular and extracellular enzymes that are largely involved in nutrient acquisition. One of the important occurrences of glycoside hydrolases in bacteria is the enzyme beta-galactosidase (LacZ), which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurofibrillary Tangles
Neurofibrillary tangles (NFTs) are aggregates of hyperphosphorylated tau protein that are most commonly known as a primary biomarker of Alzheimer's disease. Their presence is also found in numerous other diseases known as tauopathies. Little is known about their exact relationship to the different pathologies. Formation Neurofibrillary tangles are formed by hyperphosphorylation of a microtubule-associated protein known as tau, causing it to aggregate, or group, in an insoluble form. (These aggregations of hyperphosphorylated tau protein are also referred to as PHF, or " paired helical filaments"). The precise mechanism of tangle formation is not completely understood, and it is still controversial whether tangles are a primary causative factor in disease or play a more peripheral role. Cytoskeletal changes Three different maturation states of NFT have been defined using anti-tau and anti-ubiquitin immunostaining. At stage 0 there are morphologically normal pyramidal cells showi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tau Protein
The tau proteins (abbreviated from tubulin associated unit) are a group of six highly soluble protein isoforms produced by alternative splicing from the gene ''MAPT'' (microtubule-associated protein tau). They have roles primarily in maintaining the stability of microtubules in axons and are abundant in the neurons of the central nervous system (CNS), where the cerebral cortex has the highest abundance. They are less common elsewhere but are also expressed at very low levels in CNS astrocytes and oligodendrocytes. Pathologies and dementias of the nervous system such as Alzheimer's disease and Parkinson's disease are associated with tau proteins that have become hyperphosphorylated insoluble aggregates called neurofibrillary tangles. The tau proteins were identified in 1975 as heat-stable proteins essential for microtubule assembly, and since then they have been characterized as intrinsically disordered proteins. Function Microtubule stabilization Tau proteins are found mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alzheimer's Disease
Alzheimer's disease (AD) is a neurodegeneration, neurodegenerative disease that usually starts slowly and progressively worsens. It is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in short-term memory, remembering recent events. As the disease advances, symptoms can include primary progressive aphasia, problems with language, Orientation (mental), disorientation (including easily getting lost), mood swings, loss of motivation, self-neglect, and challenging behaviour, behavioral issues. As a person's condition declines, they often withdraw from family and society. Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression can vary, the typical life expectancy following diagnosis is three to nine years. The cause of Alzheimer's disease is poorly understood. There are many environmental and genetic risk factors associated with its development. The strongest genetic risk factor is from an alle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
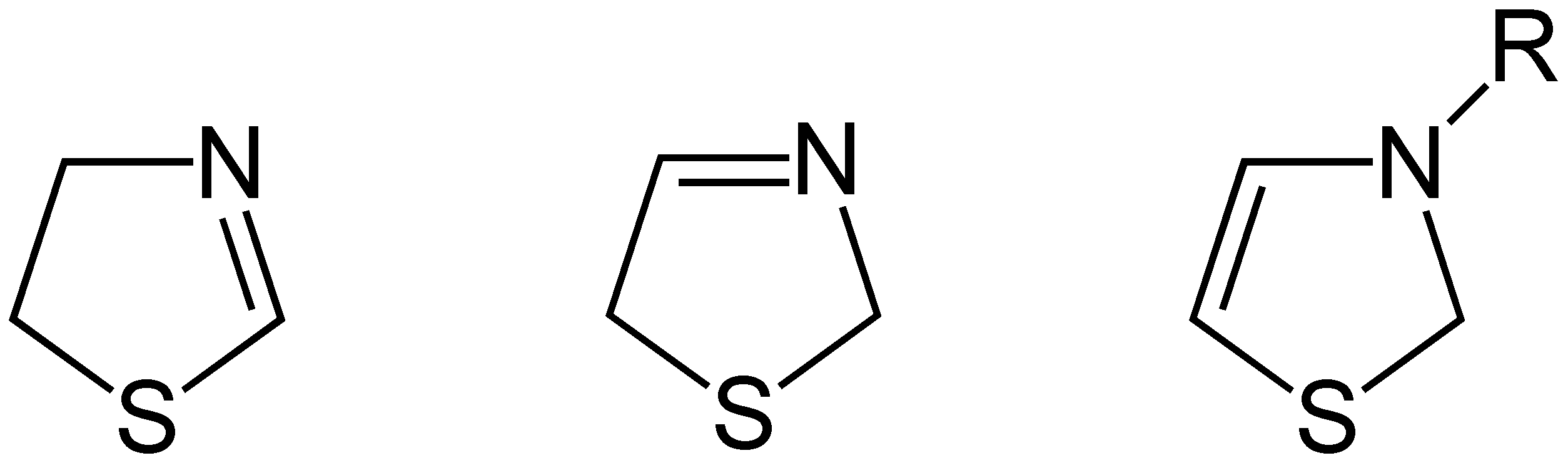
Thiazoline
Thiazolines (or dihydrothiazoles) are a group of isomeric 5-membered heterocyclic compounds containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivatives are more common and some are bioactive. For example, in a common post-translational modification, cysteine residues are converted into thiazolines. The name thiazoline originates from the Hantzsch–Widman nomenclature. Isomers Three structural isomers of thiazoline exist depending on the position of the double bond. These forms do not readily interconvert and hence are not tautomers. Of these 2-thiazoline is the most common. A fourth structure exists in which the N and S atoms are adjacent; this known as isothiazoline. Synthesis Thiazolines were first prepared by dialkylation of thioamides by Richard Willstatter in 1909. 2-Thiazolines are commonly prepared from 2-aminoethanethiols (e.g. cysteamine). They may also be synthesized via the Asinger r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond. Oxazoline itself has no applications however oxazolines have been widely investigated for potential applications. These applications include use as ligands in asymmetric catalysis, as protecting groups for carboxylic acids and increasingly as monomers for the production of polymers. Isomers Synthesis The synthesis of 2-oxazoline rings is well established and in general proceeds via the cyclisation of a 2-amino alcohol (typically obtained by the reduction of an amino acid) with a suitable functional group. The overall mechanism is usuall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
F3 Inhibitor
F3 or F03 may refer to: Computing * F3, a function key on a computer keyboard * F3 (language), the working name for JavaFX Script, a scripting language * Fat-Free Framework, a PHP web application framework Military * Douglas F-3 Havoc, a photographic reconnaissance plane * Douglas F3D Skyknight (later F-10 Skyknight), a Douglas twin engine, mid-wing jet fighter * F 3 Malmslätt, a former Swedish Air Force unit * Felixstowe F.3, a 1917 British First World War flying boat * Hannover F.3, a World War I German prototype escort fighter * HMS ''Cossack'' (F03), a 1937 British Royal Navy destroyer * HMS ''F3'', a British F class submarine * McDonnell F3H Demon (later F-3 Demon), a United States Navy carrier-based jet fighter * Mk F3 155mm, French self-propelled gun * RAF Tornado F3, a British fighter * USS ''F-3'' (SS-22), a United States Navy submarine * A future Japanese fighter jet (F-3 or F-X) based on the i3 conceptual jet fighter and Mitsubishi X-2 prototype Trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |