Nitrene on:
[Wikipedia]
[Google]
[Amazon]
 In chemistry, a nitrene or imene () is the
In chemistry, a nitrene or imene () is the
 :A nitrene intermediate is suspected in this C–H insertion involving an
:A nitrene intermediate is suspected in this C–H insertion involving an  * Nitrene cycloaddition. With
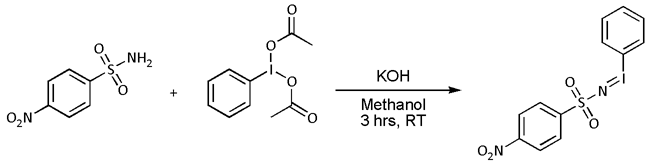
* Nitrene cycloaddition. With  :In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
::
:In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
:: :Nitrene transfer takes place next:
::
:Nitrene transfer takes place next:
:: :In this particular reaction both the ''
:In this particular reaction both the ''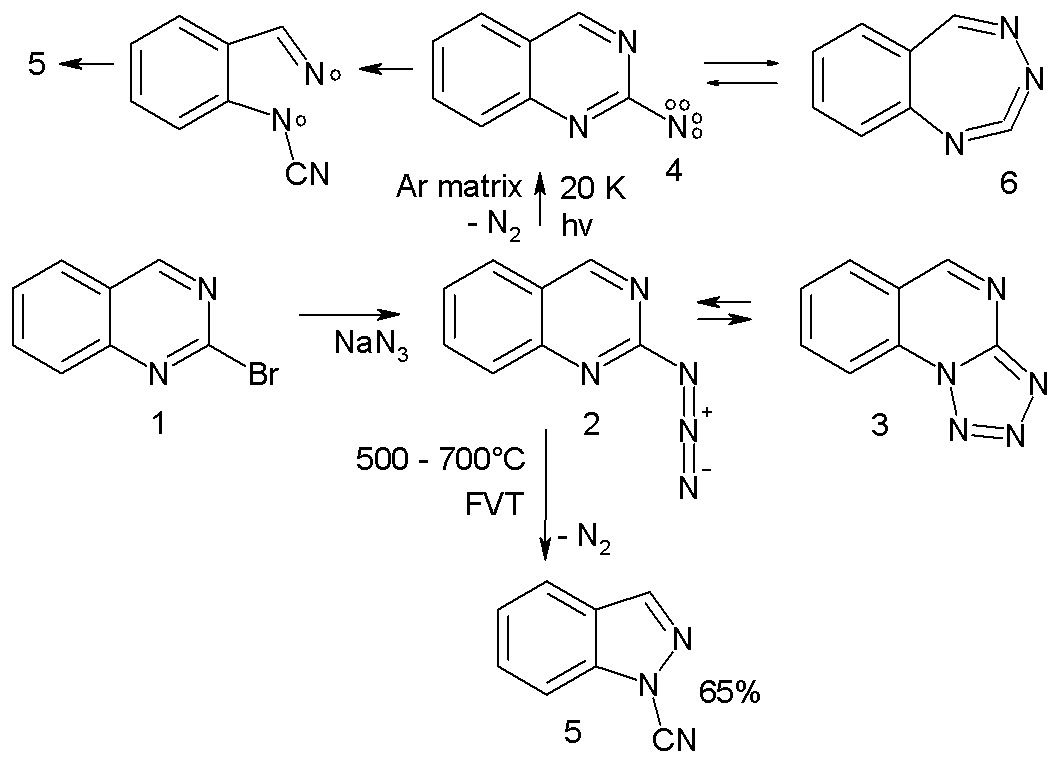 :The nitrene ultimately converts to the ring-opened nitrile 5 through the
:The nitrene ultimately converts to the ring-opened nitrile 5 through the
 In this system one of the nitrogen unpaired electrons is delocalized in the aromatic ring making the compound a σ–σ–π triradical. A
In this system one of the nitrogen unpaired electrons is delocalized in the aromatic ring making the compound a σ–σ–π triradical. A
 In chemistry, a nitrene or imene () is the
In chemistry, a nitrene or imene () is the nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
analogue of a carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
. The nitrogen atom is uncharged and univalent, so it has only 6 electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
s in its valence level—two covalent bonded and four non-bonded electrons. It is therefore considered an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
due to the unsatisfied octet. A nitrene is a reactive intermediate and is involved in many chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s. The simplest nitrene, HN, is called imidogen
Imidogen is an inorganic compound with the chemical formula NH. Like other simple radicals, it is highly reactive and consequently short-lived except as a dilute gas. Its behavior depends on its spin multiplicity.
Production and properties
Imido ...
, and that term is sometimes used as a synonym for the nitrene class.
Electron configuration
In the simplest case, the linear N–H molecule (imidogen) has its nitrogen atomsp hybridized
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
, with two of its four non-bonded electrons as a lone pair in an sp orbital and the other two occupying a degenerate
Degeneracy, degenerate, or degeneration may refer to:
Arts and entertainment
* Degenerate (album), ''Degenerate'' (album), a 2010 album by the British band Trigger the Bloodshed
* Degenerate art, a term adopted in the 1920s by the Nazi Party i ...
pair of p orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
. The electron configuration is consistent with Hund's rule
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configuration ...
: the low energy form is a triplet with one electron in each of the p orbitals and the high energy form is the singlet with an electron pair filling one p orbital and the other p orbital vacant.
As with carbenes, a strong correlation exists between the spin density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
on the nitrogen atom which can be calculated in silico and the zero-field splitting parameter ''D'' which can be derived experimentally from electron spin resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the sp ...
. Small nitrenes such as NH or CF3N have D values around 1.8 cm−1 with spin densities close to a maximum value of 2. At the lower end of the scale are molecules with low ''D'' (< 0.4) values and spin density of 1.2 to 1.4 such as 9-anthrylnitrene and 9-phenanthrylnitrene.
Formation
Because nitrenes are so reactive, they are not isolated. Instead, they are formed as reactive intermediates during a reaction. There are two common ways to generate nitrenes: * From azides bythermolysis
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
or photolysis, with expulsion of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
gas. This method is analogous to the formation of carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s from diazo compound
The diazo group is an organic moiety consisting of two linked nitrogen atoms ( azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes ...
s.
* From isocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyan ...
s, with expulsion of carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
. This method is analogous to the formation of carbenes from ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound ethen ...
s.
Reactions
Nitrene reactions include: * Nitrene C–H insertion. A nitrene can easily insert into a carbon to hydrogen covalent bond yielding an amine or amide. A singlet nitrene reacts with retention of configuration. In one study a nitrene, formed by oxidation of a carbamate withpotassium persulfate
Potassium persulfate is the inorganic compound with the formula K2 S2O8. Also known as potassium peroxydisulfate, it is a white solid that is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant, c ...
, gives an insertion reaction
An insertion reaction is a chemical reaction where one chemical entity (a molecule or molecular fragment) interposes itself into an existing bond of typically a second chemical entity ''e.g.'':
: + \longrightarrow
The term only refers to the ...
into the palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
to nitrogen bond of the reaction product of palladium(II) acetate
Palladium(II) acetate is a chemical compound of palladium described by the formula d(O2CCH3)2sub>n, abbreviated d(OAc)2sub>n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in ma ...
with 2-phenylpyridine to methyl ''N''-(2-pyridylphenyl)carbamate in a cascade reaction:
:: :A nitrene intermediate is suspected in this C–H insertion involving an
:A nitrene intermediate is suspected in this C–H insertion involving an oxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted ...
, acetic anhydride leading to an isoindole
In organic chemistry and heterocyclic chemistry, isoindole consists of a benzene ring fused with pyrrole. The compound is an isomer of indole. Its reduced form is isoindoline. The parent isoindole is a rarely encountered in the technical lite ...
:
:: * Nitrene cycloaddition. With
* Nitrene cycloaddition. With alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s, nitrenes react to form aziridines
220 px, chemotherapeutic agent by virtue of its antitumour activity. Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine (-NR-) and two methylene bridges (--). The parent compou ...
, very often with nitrenoid precursors such as nosyl- or tosyl-substituted 'N''-(phenylsulfonyl)iminohenyliodinane (PhI=NNs or PhI=NTs respectively)) but the reaction is known to work directly with the sulfonamide in presence of a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
based catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
such as copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
, or gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile me ...
:
:: :In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
::
:In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
:: :Nitrene transfer takes place next:
::
:Nitrene transfer takes place next:
:: :In this particular reaction both the ''
:In this particular reaction both the ''cis
Cis or cis- may refer to:
Places
* Cis, Trentino, in Italy
* In Poland:
** Cis, Świętokrzyskie Voivodeship, south-central
** Cis, Warmian-Masurian Voivodeship, north
Math, science and biology
* cis (mathematics) (cis(''θ'')), a trigonome ...
''-stilbene Stilbene may refer to one of the two stereoisomers of 1,2-diphenylethene:
* (''E'')-Stilbene (''trans'' isomer)
* (''Z'')-Stilbene (''cis'' isomer)
See also
* Stilbenoid
Stilbenoids are hydroxylated derivatives of stilbene. They have a C6– ...
illustrated and the ''trans'' form (not depicted) result in the same ''trans''-aziridine product, suggesting a two-step reaction mechanism. The energy difference between triplet and singlet nitrenes can be very small in some cases, allowing interconversion at room temperature. Triplet nitrenes are thermodynamically more stable but react stepwise allowing free rotation and thus producing a mixture of stereochemistry.
* Arylnitrene ring-expansion and ring-contraction: Aryl nitrenes show ring expansion to 7-membered ring cumulene
In organic chemistry, a cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is al ...
s, ring opening reactions and nitrile formations many times in complex reaction paths. For instance the azide 2 in the scheme below trapped in an argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as ...
matrix
Matrix most commonly refers to:
* ''The Matrix'' (franchise), an American media franchise
** ''The Matrix'', a 1999 science-fiction action film
** "The Matrix", a fictional setting, a virtual reality environment, within ''The Matrix'' (franchis ...
at 20 K on photolysis expels nitrogen to the triplet nitrene 4 (observed experimentally with ESR and ultraviolet-visible spectroscopy) which is in equilibrium with the ring-expansion product 6.
: :The nitrene ultimately converts to the ring-opened nitrile 5 through the
:The nitrene ultimately converts to the ring-opened nitrile 5 through the diradical
In chemistry, a diradical is a molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactive and i ...
intermediate 7. In a high-temperature reaction, FVT at 500–600 °C also yields the nitrile 5 in 65% yield.
Nitreno radicals
For several compounds containing both a nitrene group and afree radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
group an ESR high-spin quartet has been recorded (matrix, cryogenic temperatures). One of these has an amine oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that contains the functional group , a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substituent-grou ...
radical group incorporated, another system has a carbon radical group.
:carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
nitrogen radical (imidyl radical) resonance structure
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
makes a contribution to the total electronic picture.
In 2019, an authentic triplet nitrene was isolated by Betley and Lancaster, stabilized by coordination to a copper center in a bulky ligand.
References
{{Functional group Reactive intermediates Free radicals Functional groups Nitrogen compounds