|
Imidogen
Imidogen is an inorganic compound with the chemical formula NH. Like other simple radicals, it is highly reactive and consequently short-lived except as a dilute gas. Its behavior depends on its spin multiplicity. Production and properties Imidogen can be generated by electrical discharge in an atmosphere of ammonia. Imidogen has a large rotational splitting and a weak spin–spin interaction, therefore it will be less likely to undergo collision-induced Zeeman transitions. Ground-state imidogen can be magnetically trapped using buffer-gas loading from a molecular beam. The ground state of imidogen is a triplet, with a singlet excited state only slightly higher in energy. The first excited state (a1Δ) has a long lifetime as its relaxation to ground state (X3Σ−) is spin-forbidden. Imidogen undergoes collision-induced intersystem crossing. Reactivity Ignoring hydrogen atoms, imidogen is isoelectronic with carbene (CH2) and oxygen (O) atoms, and it exhibits comparable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Red Book
Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 is the 2005 version of ''Nomenclature of Inorganic Chemistry'' (which is informally called the Red Book). It is a collection of rules for naming inorganic compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). Summary The 2005 edition replaces their previous recommendations ''Nomenclature The Red Book of Inorganic Chemistry, IUPAC Recommendations 1990 (Red Book I)'', and "where appropriate" (sic) ''Nomenclature of Inorganic Chemistry II, IUPAC Recommendations 2000 (Red Book II)''. The recommendations take up over 300 pages''Nomenclature of Inorganic Chemistry IUPAC Recommendations'' 2005 ed. N. G. Connelly et al. RSC Publishing https://iupac.org/what-we-do/books/redbook/ and the full text can be downloaded from IUPAC. Corrections have been issued. Apart from a reorganisation of the content, there is a new section on organometallics and a formal element list to be used in place of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laser-induced Fluorescence
Laser-induced fluorescence (LIF) or laser-stimulated fluorescence (LSF) is a spectroscopic method in which an atom or molecule is excited to a higher energy level by the absorption of laser light followed by spontaneous emission of light. It was first reported by Zare and coworkers in 1968. LIF is used for studying structure of molecules, detection of selective species and flow visualization and measurements. The wavelength is often selected to be the one at which the species has its largest cross section. The excited species will after some time, usually in the order of few nanoseconds to microseconds, de-excite and emit light at a wavelength longer than the excitation wavelength. This fluorescent light is typically recorded with a photomultiplier tube (PMT) or filtered photodiodes. Types Two different kinds of spectra exist, disperse spectra and excitation spectra. The disperse spectra are performed with a fixed lasing wavelength, as above and the fluorescence spectrum is anal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
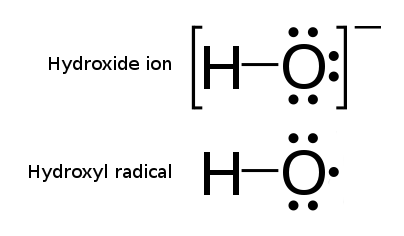
Hydroxyl Radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of 1-hydroxy-2(1''H'')-pyridinethione. Notation The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O. Biology Hydroxyl radicals can occasionally be produced as a byproduct of immune action. Macrophages and microglia most frequently generate this compound when exp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astrophysical Journal Letters
''The Astrophysical Journal'', often abbreviated ''ApJ'' (pronounced "ap jay") in references and speech, is a Peer review, peer-reviewed scientific journal of astrophysics and astronomy, established in 1895 by American astronomers George Ellery Hale and James Edward Keeler. The journal discontinued its print edition and became an electronic-only journal in 2015. Since 1953 ''The Astrophysical Journal Supplement Series'' (''ApJS'') has been published in conjunction with ''The Astrophysical Journal'', with generally longer articles to supplement the material in the journal. It publishes six volumes per year, with two 280-page issues per volume. ''The Astrophysical Journal Letters'' (''ApJL''), established in 1967 by Subrahmanyan Chandrasekhar as Part 2 of ''The Astrophysical Journal'', is now a separate journal focusing on the rapid publication of high-impact astronomical research. The three journals were published by the University of Chicago Press for the American Astronomical S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoionization
Photoionization is the physical process in which an ion is formed from the interaction of a photon with an atom or molecule. Cross section Not every interaction between a photon and an atom, or molecule, will result in photoionization. The probability of photoionization is related to the photoionization cross section of the species -- the probability of an ionization event conceptualized as a hypothetical cross-sectional area. This cross section depends on the energy of the photon (proportional to its wavenumber) and the species being considered i.e. it depends on the structure of the molecular species. In the case of molecules, the photoionization cross-section can be estimated by examination of Franck-Condon factors between a ground-state molecule and the target ion. This can be initialized by computing the vibrations of a molecule and associated cation (post ionization) using quantum chemical software e.g. QChem. For photon energies below the ionization threshold, the photoion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. Photodissociation is not limited to visible light. Any photon with sufficient energy can affect the chemical bonds of a chemical compound. Since a photon's energy is inversely proportional to its wavelength, electromagnetic radiations with the energy of visible light or higher, such as ultraviolet light, x-rays, and gamma rays can induce such reactions. Photolysis in photosynthesis Photolysis is part of the light-dependent reaction or light phase or photochemical phase or Hill reaction of photosynthesis. The general reaction of photosynthetic photolysis can be given in terms of photons as: :\ce + 2 \text \longrightarrow \ce The chemical nature of "A" depends on the type of organism. Purple sulfur bacteria oxidize hydrogen sulfide () ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monthly Notices Of The Royal Astronomical Society
''Monthly Notices of the Royal Astronomical Society'' (MNRAS) is a peer-reviewed scientific journal covering research in astronomy and astrophysics. It has been in continuous existence since 1827 and publishes letters and papers reporting original research in relevant fields. Despite the name, the journal is no longer monthly, nor does it carry the notices of the Royal Astronomical Society. History The first issue of MNRAS was published on 9 February 1827 as ''Monthly Notices of the Astronomical Society of London'' and it has been in continuous publication ever since. It took its current name from the second volume, after the Astronomical Society of London became the Royal Astronomical Society (RAS). Until 1960 it carried the monthly notices of the RAS, at which time these were transferred to the newly established ''Quarterly Journal of the Royal Astronomical Society'' (1960–1996) and then to its successor journal ''Astronomy & Geophysics'' (since 1997). Until 1965, MNRAS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal-to-noise
Signal-to-noise ratio (SNR or S/N) is a measure used in science and engineering that compares the level of a desired signal to the level of background noise. SNR is defined as the ratio of signal power to the noise power, often expressed in decibels. A ratio higher than 1:1 (greater than 0 dB) indicates more signal than noise. SNR, bandwidth, and channel capacity of a communication channel are connected by the Shannon–Hartley theorem. Definition Signal-to-noise ratio is defined as the ratio of the power of a signal (meaningful input) to the power of background noise (meaningless or unwanted input): : \mathrm = \frac, where is average power. Both signal and noise power must be measured at the same or equivalent points in a system, and within the same system bandwidth. Depending on whether the signal is a constant () or a random variable (), the signal-to-noise ratio for random noise becomes: : \mathrm = \frac where E refers to the expected value, i.e. in this case th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ζ Persei
Zeta Persei (ζ Per, ζ Persei) is a star in the northern constellation of Perseus. With an apparent visual magnitude of 2.9, it can be readily seen with the naked eye. Parallax measurements place it at a distance of about from Earth. Description This is a lower luminosity supergiant star with a stellar classification of B1 Ib. This is an enormous star, with an estimated 26–27 times the Sun's radius and 13–16 times the Sun's mass. It has about 47,000 times the luminosity of the Sun and it is radiating this energy at an effective temperature of 20,800 K, giving it the blue-white hue of a B-type star. The spectrum displays anomalously high levels of carbon. Zeta Persei has a strong stellar wind that is expelling times the mass of the Sun per year, or the equivalent of the Sun's mass every 4.3 million years. Zeta Persei has a 9th magnitude companion at an angular separation of 12.9 arcseconds. The two stars have the same proper motion, so they may be phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Preferred IUPAC Name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations. Preferred IUPAC names are applicable only for organic compounds, to which the IUPAC has the definition as compounds which contain at least a single carbon atom but no alkali, alkaline earth or transition metals and can be named by the nomenclature of organic compounds (see below). Rules for the remaining organic and inorganic compounds are still under development. The concept of PINs is defined in the introductory chapter (freely accessible) and chapter 5 of the ''"Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013"'', which replace two former publicat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |