Nickel Boride (other) on:
[Wikipedia]
[Google]
[Amazon]
Nickel is a chemical element with
 Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are
Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are
To read the nickel atom levels, type "Ni I" in the Spectrum box and click on Retrieve data. However, each of these two configurations splits into several energy levels due to
 On Earth, nickel occurs most often in combination with
On Earth, nickel occurs most often in combination with

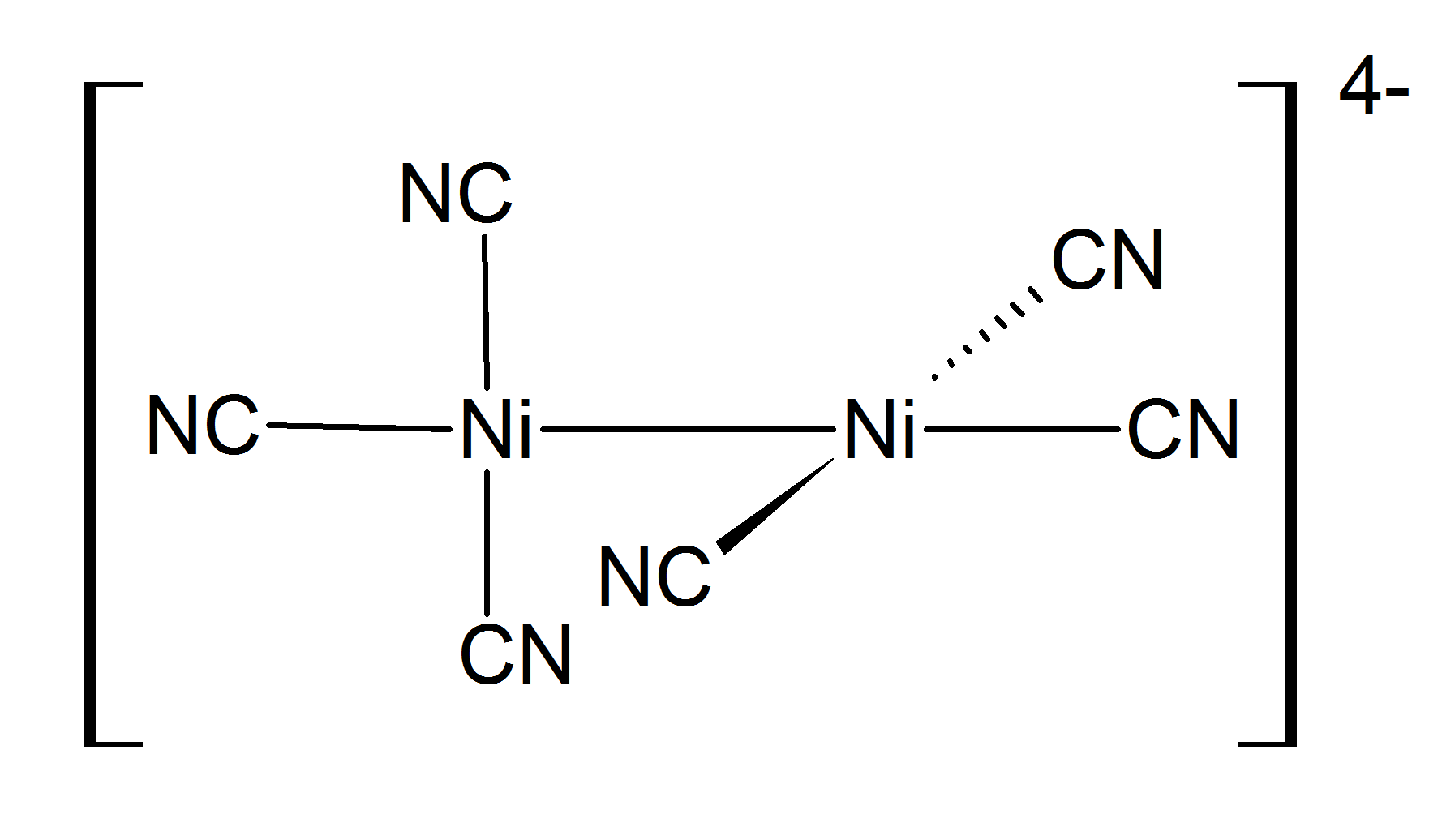 Nickel(I) complexes are uncommon, but one example is the tetrahedral complex . Many nickel(I) complexes have Ni–Ni bonding, such as the dark red diamagnetic prepared by reduction of with sodium amalgam. This compound is oxidized in water, liberating .
It is thought that the nickel(I) oxidation state is important to nickel-containing enzymes, such as iFehydrogenase, which catalyzes the reversible reduction of protons to .
Nickel(I) complexes are uncommon, but one example is the tetrahedral complex . Many nickel(I) complexes have Ni–Ni bonding, such as the dark red diamagnetic prepared by reduction of with sodium amalgam. This compound is oxidized in water, liberating .
It is thought that the nickel(I) oxidation state is important to nickel-containing enzymes, such as iFehydrogenase, which catalyzes the reversible reduction of protons to .

 Nickel(II) forms compounds with all common anions, including
Nickel(II) forms compounds with all common anions, including
 Many Ni(III) compounds are known. The first such compounds are , where X = Cl, Br, I and R =
Many Ni(III) compounds are known. The first such compounds are , where X = Cl, Br, I and R =
 In medieval Germany, a metallic yellow mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. But when miners were unable to get any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to '' Old Nick''), for besetting the copper. They called this ore ''Kupfernickel'' from German ''Kupfer'' 'copper'. This ore is now known as the mineral nickeline (formerly ''niccolite''), a nickel arsenide. In 1751, Baron Axel Fredrik Cronstedt tried to extract copper from kupfernickel at a cobalt mine in the village of
In medieval Germany, a metallic yellow mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. But when miners were unable to get any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to '' Old Nick''), for besetting the copper. They called this ore ''Kupfernickel'' from German ''Kupfer'' 'copper'. This ore is now known as the mineral nickeline (formerly ''niccolite''), a nickel arsenide. In 1751, Baron Axel Fredrik Cronstedt tried to extract copper from kupfernickel at a cobalt mine in the village of
 Aside from the aforementioned Bactrian coins, nickel was not a component of coins until the mid-19th century.
Aside from the aforementioned Bactrian coins, nickel was not a component of coins until the mid-19th century.
 In the United States, the term "nickel" or "nick" originally applied to the copper-nickel Flying Eagle cent, which replaced copper with 12% nickel 1857–58, then the Indian Head cent of the same alloy from 1859 to 1864. Still later, in 1865, the term designated the three-cent nickel, with nickel increased to 25%. In 1866, the five-cent shield nickel (25% nickel, 75% copper) appropriated the designation, which has been used ever since for the subsequent 5-cent pieces. This alloy proportion is not
In the United States, the term "nickel" or "nick" originally applied to the copper-nickel Flying Eagle cent, which replaced copper with 12% nickel 1857–58, then the Indian Head cent of the same alloy from 1859 to 1864. Still later, in 1865, the term designated the three-cent nickel, with nickel increased to 25%. In 1866, the five-cent shield nickel (25% nickel, 75% copper) appropriated the designation, which has been used ever since for the subsequent 5-cent pieces. This alloy proportion is not

 An estimated 2.7 million tonnes (t) of nickel per year are mined worldwide; Indonesia (1,000,000 t), the Philippines (370,000 t), Russia (250,000 t),
An estimated 2.7 million tonnes (t) of nickel per year are mined worldwide; Indonesia (1,000,000 t), the Philippines (370,000 t), Russia (250,000 t),
 Nickel is obtained through extractive metallurgy: it is extracted from ore by conventional roasting and reduction processes that yield metal of greater than 75% purity. In many
Nickel is obtained through extractive metallurgy: it is extracted from ore by conventional roasting and reduction processes that yield metal of greater than 75% purity. In many 
 The purest metal is obtained from nickel oxide by the Mond process, which gives a purity of over 99.99%. The process was patented by Ludwig Mond and has been in industrial use since before the beginning of the 20th century. In this process, nickel is reacted with carbon monoxide in the presence of a sulfur catalyst at around 40–80 °C to form nickel carbonyl. In a similar reaction with iron, iron pentacarbonyl can form, though this reaction is slow. If necessary, the nickel may be separated by distillation.
The purest metal is obtained from nickel oxide by the Mond process, which gives a purity of over 99.99%. The process was patented by Ludwig Mond and has been in industrial use since before the beginning of the 20th century. In this process, nickel is reacted with carbon monoxide in the presence of a sulfur catalyst at around 40–80 °C to form nickel carbonyl. In a similar reaction with iron, iron pentacarbonyl can form, though this reaction is slow. If necessary, the nickel may be separated by distillation.
 Global use of nickel is currently 68% in stainless steel, 10% in nonferrous alloys, 9%
Global use of nickel is currently 68% in stainless steel, 10% in nonferrous alloys, 9%  Because nickel is resistant to corrosion, it was occasionally used as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above), but in the later years of the 20th century, it was replaced by cheaper
Because nickel is resistant to corrosion, it was occasionally used as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above), but in the later years of the 20th century, it was replaced by cheaper
"Nickel and nickel compounds"
in ''IARC Monogr Eval Carcinog Risks Hum''. Volume 100C. pp. 169–218..Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006 J L 353, 31.12.2008, p. 1br>Annex VI
. Accessed July 13, 2017.Globally Harmonised System of Classification and Labelling of Chemicals (GHS)
, 5th ed., United Nations, New York and Geneva, 2013..National Toxicology Program. (2016)
, 14th ed. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service.. based on increased respiratory cancer risks observed in epidemiological studies of sulfidic ore refinery workers. This is supported by the positive results of the NTP bioassays with Ni sub-sulfide and Ni oxide in rats and mice. The human and animal data consistently indicate a lack of carcinogenicity via the oral route of exposure and limit the carcinogenicity of nickel compounds to respiratory tumours after inhalation. Nickel metal is classified as a suspect carcinogen; there is consistency between the absence of increased respiratory cancer risks in workers predominantly exposed to metallic nickel and the lack of respiratory tumours in a rat lifetime inhalation carcinogenicity study with nickel metal powder. In the rodent inhalation studies with various nickel compounds and nickel metal, increased lung inflammations with and without bronchial lymph node hyperplasia or fibrosis were observed. In rat studies, oral ingestion of water-soluble nickel salts can trigger perinatal mortality in pregnant animals. Whether these effects are relevant to humans is unclear as epidemiological studies of highly exposed female workers have not shown adverse developmental toxicity effects. People can be exposed to nickel in the workplace by inhalation, ingestion, and contact with skin or eye. The Occupational Safety and Health Administration (OSHA) has set the legal limit ( permissible exposure limit) for the workplace at 1 mg/m per 8-hour workday, excluding nickel carbonyl. The National Institute for Occupational Safety and Health (NIOSH) sets the recommended exposure limit (REL) at 0.015 mg/m per 8-hour workday. At 10 mg/m, nickel is
. American Academy of Dermatology(August 22, 2015) Reports show that both the nickel-induced activation of hypoxia-inducible factor (HIF-1) and the up-regulation of hypoxia-inducible genes are caused by depletion of intracellular
Nickel
at '' The Periodic Table of Videos'' (University of Nottingham)
CDC – Nickel – NIOSH Workplace Safety and Health Topic
An occupational hygiene assessment of dermal nickel exposures in primary production industries
by GW Hughson. Institute of Occupational Medicine Research Report TM/04/05
An occupational hygiene assessment of dermal nickel exposures in primary production and primary user industries. Phase 2 Report
by GW Hughson. Institute of Occupational Medicine Research Report TM/05/06
"The metal that brought you cheap flights"
BBC News {{Authority control Chemical elements Transition metals Dietary minerals Ferromagnetic materials IARC Group 2B carcinogens Native element minerals Chemical elements with face-centered cubic structure
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere.
Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis
Supernova nucleosynthesis is the nucleosynthesis of chemical elements in supernova explosions.
In sufficiently massive stars, the nucleosynthesis by fusion of lighter elements into heavier ones occurs during sequential hydrostatic burning processe ...
. An iron–nickel mixture is thought to compose Earth's outer and inner cores.
Use of nickel (as natural meteoric
A meteoroid () is a small rocky or metallic body in outer space.
Meteoroids are defined as objects significantly smaller than asteroids, ranging in size from grains to objects up to a meter wide. Objects smaller than this are classified as micr ...
nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an element in 1751 by Axel Fredrik Cronstedt, who initially mistook the ore for a copper mineral, in the cobalt mines of Los, Hälsingland, Sweden. The element's name comes from a mischievous sprite of German miner mythology, Nickel (similar to Old Nick), who personified the fact that copper-nickel ores resisted refinement into copper. An economically important source of nickel is the iron ore limonite
Limonite () is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as FeO(OH)·H2O, although this is not entirely accurate as the ratio of oxide to hydroxid ...
, which is often 1–2% nickel. Other important nickel ore minerals include pentlandite and a mix of Ni-rich natural silicates known as garnierite. Major production sites include the Sudbury region, Canada (which is thought to be of meteoric
A meteoroid () is a small rocky or metallic body in outer space.
Meteoroids are defined as objects significantly smaller than asteroids, ranging in size from grains to objects up to a meter wide. Objects smaller than this are classified as micr ...
origin), New Caledonia
)
, anthem = ""
, image_map = New Caledonia on the globe (small islands magnified) (Polynesia centered).svg
, map_alt = Location of New Caledonia
, map_caption = Location of New Caledonia
, mapsize = 290px
, subdivision_type = Sovereign st ...
in the Pacific, and Norilsk, Russia.
Nickel is one of four elements (the others are iron, cobalt, and gadolinium) that are ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
at about room temperature. Alnico permanent magnets based partly on nickel are of intermediate strength between iron-based permanent magnets and rare-earth magnet
Rare-earth magnets are strong permanent magnets made from alloys of rare-earth elements. Developed in the 1970s and 1980s, rare-earth magnets are the strongest type of permanent magnets made, producing significantly stronger magnetic fields than ...
s. The metal is used chiefly in alloys and corrosion-resistant plating. About 68% of world production is used in stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corros ...
. A further 10% is used for nickel-based and copper-based alloys, 9% for plating, 7% for alloy steels, 3% in foundries, and 4% in other applications such as in rechargeable batteries, including those in electric vehicle
An electric vehicle (EV) is a vehicle that uses one or more electric motors for propulsion. It can be powered by a collector system, with electricity from extravehicular sources, or it can be powered autonomously by a battery (sometimes cha ...
s (EVs). Nickel is widely used in coins, though nickel-plated objects sometimes provoke nickel allergy. As a compound, nickel has a number of niche chemical manufacturing uses, such as a catalyst for hydrogenation, cathodes for rechargeable batteries, pigments and metal surface treatments. Nickel is an essential nutrient for some microorganisms and plants that have enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
with nickel as an active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
.
Properties
Atomic and physical properties
 Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are
Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
at or near room temperature; the others are iron, cobalt and gadolinium. Its Curie temperature is , meaning that bulk nickel is non-magnetic above this temperature. The unit cell of nickel is a face-centered cube with the lattice parameter of 0.352 nm, giving an atomic radius of 0.124 nm. This crystal structure is stable to pressures of at least 70 GPa. Nickel is hard, malleable and ductile, and has a relatively high electrical and thermal conductivity for transition metals. The high compressive strength
In mechanics, compressive strength or compression strength is the capacity of a material or structure to withstand loads tending to reduce size (as opposed to tensile strength which withstands loads tending to elongate). In other words, compre ...
of 34 GPa, predicted for ideal crystals, is never obtained in the real bulk material due to formation and movement of dislocations. However, it has been reached in Ni nanoparticles.
Electron configuration dispute
Nickel has two atomicelectron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
s, r3d 4s and r3d 4s, which are very close in energy; rdenotes the complete argon core structure. There is some disagreement on which configuration has the lower energy. Chemistry textbooks quote nickel's electron configuration as r4s 3d, also written r3d 4s. This configuration agrees with the Madelung energy ordering rule, which predicts that 4s is filled before 3d. It is supported by the experimental fact that the lowest energy state of the nickel atom is a 3d 4s energy level, specifically the 3d(F) 4s F, ''J'' = 4 level.NIST Atomic Spectrum DatabaseTo read the nickel atom levels, type "Ni I" in the Spectrum box and click on Retrieve data. However, each of these two configurations splits into several energy levels due to
fine structure
In atomic physics, the fine structure describes the splitting of the spectral lines of atoms due to electron spin and relativistic corrections to the non-relativistic Schrödinger equation. It was first measured precisely for the hydrogen atom ...
, and the two sets of energy levels overlap. The average energy of states with r3d 4s is actually lower than the average energy of states with r3d 4s. Therefore, the research literature on atomic calculations quotes the ground state configuration as r3d 4s.
Isotopes
The isotopes of nickel range in atomic weight from 48 u () to 78 u (). Natural nickel is composed of five stable isotopes, , , , and , of which is the most abundant (68.077% natural abundance). Nickel-62 has the highest binding energy per nucleon of anynuclide
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by Truman ...
: 8.7946 MeV/nucleon. Its binding energy is greater than both and , more abundant nuclides often incorrectly cited as having the highest binding energy. Though this would seem to predict nickel as the most abundant heavy element in the universe, the high rate of photodisintegration of nickel in stellar interiors causes iron to be by far the most abundant.
Nickel-60 is the daughter product of the extinct radionuclide (half-life 2.6 million years). Due to the long half-life of , its persistence in materials in the Solar System may generate observable variations in the isotopic composition of . Therefore, the abundance of in extraterrestrial material may give insight into the origin of the Solar System and its early history.
At least 26 nickel radioisotopes have been characterized; the most stable are with half-life 76,000 years, (100 years), and (6 days). All other radioisotopes have half-lives less than 60 hours and most these have half-lives less than 30 seconds. This element also has one meta state.
Radioactive nickel-56 is produced by the silicon burning process and later set free in large amounts in type Ia supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when ...
e. The shape of the light curve of these supernovae at intermediate to late-times corresponds to the decay via electron capture of to cobalt-56 and ultimately to iron-56. Nickel-59 is a long-lived cosmogenic radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transfer ...
; half-life 76,000 years. has found many applications in isotope geology. has been used to date the terrestrial age of meteorite
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or Natural satellite, moon. When the ...
s and to determine abundances of extraterrestrial dust in ice and sediment. The half-life of nickel-78 was recently measured at 110 milliseconds, and is believed an important isotope in supernova nucleosynthesis
Supernova nucleosynthesis is the nucleosynthesis of chemical elements in supernova explosions.
In sufficiently massive stars, the nucleosynthesis by fusion of lighter elements into heavier ones occurs during sequential hydrostatic burning processe ...
of elements heavier than iron. Ni, discovered in 1999, is the most proton-rich heavy element isotope known. With 28 proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s and 20 neutrons, Ni is " doubly magic", as is Ni with 28 protons and 50 neutrons. Both are therefore unusually stable for nuclei with so large a proton–neutron imbalance.
Nickel-63 is a contaminant found in the support structure of nuclear reactors. It is produced through neutron capture by nickel-62. Small amounts have also been found near nuclear weapon test sites in the South Pacific.
Occurrence
 On Earth, nickel occurs most often in combination with
On Earth, nickel occurs most often in combination with sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
and iron in pentlandite, with sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
in millerite, with arsenic in the mineral nickeline, and with arsenic and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
in nickel galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It cryst ...
. Nickel is commonly found in iron meteorites as the alloys kamacite and taenite. Nickel in meteorites was first detected in 1799 by Joseph-Louis Proust, a French chemist who then worked in Spain. Proust analyzed samples of the meteorite from Campo del Cielo (Argentina), which had been obtained in 1783 by Miguel Rubín de Celis, discovering the presence in them of nickel (about 10%) along with iron.
The bulk of nickel is mined
Mined may refer to:
* Mined (text editor), a terminal-based text editor
* Mining, the extraction of valuable geological materials from the Earth
See also
* Mind (disambiguation)
* Mine (disambiguation)
Mine, mines, miners or mining may refer ...
from two types of ore deposits. The first is laterite
Laterite is both a soil and a rock type rich in iron and aluminium and is commonly considered to have formed in hot and wet tropical areas. Nearly all laterites are of rusty-red coloration, because of high iron oxide content. They develop by ...
, where the principal ore mineral mixtures are nickeliferous limonite
Limonite () is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as FeO(OH)·H2O, although this is not entirely accurate as the ratio of oxide to hydroxid ...
, (Fe,Ni)O(OH), and garnierite (a mixture of various hydrous nickel and nickel-rich silicates). The second is magmatic sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
deposits, where the principal ore mineral is pentlandite: .
Indonesia and Australia
Australia, officially the Commonwealth of Australia, is a Sovereign state, sovereign country comprising the mainland of the Australia (continent), Australian continent, the island of Tasmania, and numerous List of islands of Australia, sma ...
have the biggest estimated reserves, at 43.6% of world total.
Identified land-based resources throughout the world averaging 1% nickel or greater comprise at least 130 million tons of nickel (about the double of known reserves). About 60% is in laterites and 40% in sulfide deposits.
On geophysical evidence, most of the nickel on Earth is believed to be in Earth's outer and inner cores. Kamacite and taenite are naturally occurring alloys of iron and nickel. For kamacite, the alloy is usually in the proportion of 90:10 to 95:5, though impurities (such as cobalt or carbon) may be present. Taenite is 20% to 65% nickel. Kamacite and taenite are also found in nickel iron meteorites.
Compounds
The most common oxidation state of nickel is +2, but compounds of , , and are well known, and the exotic oxidation states and have been produced and studied.Nickel(0)

Nickel tetracarbonyl
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-pur ...
), discovered by Ludwig Mond, is a volatile, highly toxic liquid at room temperature. On heating, the complex decomposes back to nickel and carbon monoxide:
:
This behavior is exploited in the Mond process for purifying nickel, as described above. The related nickel(0) complex bis(cyclooctadiene)nickel(0)
Bis(cyclooctadiene)nickel(0) is the organonickel compound with the formula Ni(C8H12)2, also written Ni(cod)2. It is a diamagnetic coordination complex featuring tetrahedral nickel(0) bound to the alkene groups in two 1,5-cyclooctadiene ligands. T ...
is a useful catalyst in organonickel chemistry
Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel com ...
because the cyclooctadiene
A cyclooctadiene (sometimes abbreviated COD) is any of several cyclic diene with the formula (CH2)4(C2H2)2. Focusing only on cis derivatives, four isomers are possible: 1,2-, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers ar ...
(or ''cod'') ligands are easily displaced.
Nickel(I)
 Nickel(I) complexes are uncommon, but one example is the tetrahedral complex . Many nickel(I) complexes have Ni–Ni bonding, such as the dark red diamagnetic prepared by reduction of with sodium amalgam. This compound is oxidized in water, liberating .
It is thought that the nickel(I) oxidation state is important to nickel-containing enzymes, such as iFehydrogenase, which catalyzes the reversible reduction of protons to .
Nickel(I) complexes are uncommon, but one example is the tetrahedral complex . Many nickel(I) complexes have Ni–Ni bonding, such as the dark red diamagnetic prepared by reduction of with sodium amalgam. This compound is oxidized in water, liberating .
It is thought that the nickel(I) oxidation state is important to nickel-containing enzymes, such as iFehydrogenase, which catalyzes the reversible reduction of protons to .
Nickel(II)

 Nickel(II) forms compounds with all common anions, including
Nickel(II) forms compounds with all common anions, including sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
, sulfate, carbonate, hydroxide, carboxylates, and halides. Nickel(II) sulfate is produced in large amounts by dissolving nickel metal or oxides in sulfuric acid, forming both a hexa- and heptahydrateLascelles, Keith; Morgan, Lindsay G.; Nicholls, David and Beyersmann, Detmar (2019) "Nickel Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry''. Wiley-VCH, Weinheim. useful for electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be ...
nickel. Common salts of nickel, such as chloride, nitrate, and sulfate, dissolve in water to give green solutions of the metal aquo complex .
The four halides form nickel compounds, which are solids with molecules with octahedral Ni centres. Nickel(II) chloride is most common, and its behavior is illustrative of the other halides. Nickel(II) chloride is made by dissolving nickel or its oxide in hydrochloric acid. It is usually found as the green hexahydrate, whose formula is usually written . When dissolved in water, this salt forms the metal aquo complex . Dehydration of gives yellow anhydrous .
Some tetracoordinate nickel(II) complexes, e.g. bis(triphenylphosphine)nickel chloride
Dichlorobis(triphenylphosphine)nickel(II) refers to a pair of metal phosphine complexes with the formula NiCl2 (C6H5)3sub>2. The compound exists as two isomers, a paramagnetic dark blue solid and a diamagnetic red solid. These complexes function a ...
, exist both in tetrahedral and square planar geometries. The tetrahedral complexes are paramagnetic; the square planar complexes are diamagnetic. In having properties of magnetic equilibrium and formation of octahedral complexes, they contrast with the divalent complexes of the heavier group 10 metals, palladium(II) and platinum(II), which form only square-planar geometry.
Nickelocene is known; it has an electron count of 20, making it relatively unstable.
Nickel(III) and (IV)
 Many Ni(III) compounds are known. The first such compounds are , where X = Cl, Br, I and R =
Many Ni(III) compounds are known. The first such compounds are , where X = Cl, Br, I and R = ethyl
Ethyl may refer to:
Arts and entertainment
* Cold Ethyl, a Swedish rock band
*Ethyl Sinclair, a character in the ''Dinosaurs'' television show
Science and technology
* Ethyl group, an organic chemistry moiety
* Ethyl alcohol (or ethanol)
* E ...
, propyl, butyl. Further, Ni(III) forms simple salts with fluoride or oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
ions. Ni(III) can be stabilized by σ-donor ligands such as thiols and organophosphines.
Ni(III) occurs in nickel oxide hydroxide, which is used as the cathode in many rechargeable batteries, including nickel-cadmium, nickel-iron, nickel hydrogen, and nickel-metal hydride, and used by certain manufacturers in Li-ion
A lithium-ion or Li-ion battery is a type of rechargeable battery which uses the reversible Redox, reduction of lithium ions to store energy. It is the predominant battery type used in portable consumer electronics and electric vehicles. It als ...
batteries.
Ni(IV) occurs in the mixed oxide . Ni(IV) remains a rare oxidation state and very few compounds are known.
History
Because nickel ores are easily mistaken for ores of silver and copper, understanding of this metal and its use, is relatively recent. But unintentional use of nickel is ancient, and can be traced back as far as 3500 BCE.Bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such ...
s from what is now Syria have been found to contain as much as 2% nickel. Some ancient Chinese manuscripts suggest that "white copper" ( cupronickel, known as ''baitong'') was used there in 1700-1400 BCE. This Paktong white copper was exported to Britain as early as the 17th century, but the nickel content of this alloy was not discovered until 1822. Coins of nickel-copper alloy were minted by Bactrian kings Agathocles, Euthydemus II, and Pantaleon in the 2nd century BCE, possibly out of the Chinese cupronickel.
 In medieval Germany, a metallic yellow mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. But when miners were unable to get any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to '' Old Nick''), for besetting the copper. They called this ore ''Kupfernickel'' from German ''Kupfer'' 'copper'. This ore is now known as the mineral nickeline (formerly ''niccolite''), a nickel arsenide. In 1751, Baron Axel Fredrik Cronstedt tried to extract copper from kupfernickel at a cobalt mine in the village of
In medieval Germany, a metallic yellow mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. But when miners were unable to get any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to '' Old Nick''), for besetting the copper. They called this ore ''Kupfernickel'' from German ''Kupfer'' 'copper'. This ore is now known as the mineral nickeline (formerly ''niccolite''), a nickel arsenide. In 1751, Baron Axel Fredrik Cronstedt tried to extract copper from kupfernickel at a cobalt mine in the village of Los, Sweden
Los is a locality situated in Ljusdal Municipality, Gävleborg County, Sweden with 332 inhabitants in 2020.
The village is known for its 18th-century cobalt mine, where Axel Fredrik Cronstedt discovered the chemical element of nickel
Nickel ...
, and instead produced a white metal that he named ''nickel'' after the spirit that had given its name to the mineral. In modern German, Kupfernickel or Kupfer-Nickel designates the alloy cupronickel.
Originally, the only source for nickel was the rare Kupfernickel. Beginning in 1824, nickel was obtained as a byproduct of cobalt blue
Cobalt blue is a blue pigment made by sintering cobalt(II) oxide with aluminum(III) oxide (alumina) at 1200 °C. Chemically, cobalt blue pigment is cobalt(II) oxide-aluminium oxide, or cobalt(II) aluminate, CoAl2O4. Cobalt blue is lighter ...
production. The first large-scale smelting of nickel began in Norway in 1848 from nickel-rich pyrrhotite. The introduction of nickel in steel production in 1889 increased the demand for nickel; the nickel deposits of New Caledonia
)
, anthem = ""
, image_map = New Caledonia on the globe (small islands magnified) (Polynesia centered).svg
, map_alt = Location of New Caledonia
, map_caption = Location of New Caledonia
, mapsize = 290px
, subdivision_type = Sovereign st ...
, discovered in 1865, provided most of the world's supply between 1875 and 1915. The discovery of the large deposits in the Sudbury Basin
The Sudbury Basin (), also known as Sudbury Structure or the Sudbury Nickel Irruptive, is a major geological structure in Ontario, Canada. It is the third-largest known impact crater or astrobleme on Earth, as well as one of the oldest. The cra ...
, Canada in 1883, in Norilsk-Talnakh, Russia in 1920, and in the Merensky Reef, South Africa in 1924, made large-scale nickel production possible.
Coinage
 Aside from the aforementioned Bactrian coins, nickel was not a component of coins until the mid-19th century.
Aside from the aforementioned Bactrian coins, nickel was not a component of coins until the mid-19th century.
Canada
99.9% nickel five-cent coins were struck in Canada (the world's largest nickel producer at the time) during non-war years from 1922 to 1981; the metal content made these coins magnetic. During the war years 1942–45, most or all nickel was removed from Canadian and US coins to save it for making armor. Canada used 99.9% nickel from 1968 in its higher-value coins until 2000.Switzerland
Coins of nearly pure nickel were first used in 1881 in Switzerland.United Kingdom
Birmingham forged nickel coins in for trading in Malaysia.United States
 In the United States, the term "nickel" or "nick" originally applied to the copper-nickel Flying Eagle cent, which replaced copper with 12% nickel 1857–58, then the Indian Head cent of the same alloy from 1859 to 1864. Still later, in 1865, the term designated the three-cent nickel, with nickel increased to 25%. In 1866, the five-cent shield nickel (25% nickel, 75% copper) appropriated the designation, which has been used ever since for the subsequent 5-cent pieces. This alloy proportion is not
In the United States, the term "nickel" or "nick" originally applied to the copper-nickel Flying Eagle cent, which replaced copper with 12% nickel 1857–58, then the Indian Head cent of the same alloy from 1859 to 1864. Still later, in 1865, the term designated the three-cent nickel, with nickel increased to 25%. In 1866, the five-cent shield nickel (25% nickel, 75% copper) appropriated the designation, which has been used ever since for the subsequent 5-cent pieces. This alloy proportion is not ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
.
The US nickel coin contains of nickel, which at the April 2007 price was worth 6.5 cents, along with 3.75 grams of copper worth about 3 cents, with a total metal value of more than 9 cents. Since the face value of a nickel is 5 cents, this made it an attractive target for melting by people wanting to sell the metals at a profit. The United States Mint, anticipating this practice, implemented new interim rules on December 14, 2006, subject to public comment for 30 days, which criminalized the melting and export of cents and nickels. Violators can be punished with a fine of up to $10,000 and/or a maximum of five years in prison. As of September 19, 2013, the melt value of a US nickel (copper and nickel included) is $0.045 (90% of the face value).
Current use
In the 21st century, the high price of nickel has led to some replacement of the metal in coins around the world. Coins still made with nickel alloys include one- and two- euro coins, 5¢, 10¢, 25¢, 50¢, and $1 U.S. coins, and 20p, 50p, £1, and £2 UK coins. From 2012 on the nickel-alloy used for 5p and 10p UK coins was replaced with nickel-plated steel. This ignited a public controversy regarding the problems of people with nickel allergy.World production
New Caledonia
)
, anthem = ""
, image_map = New Caledonia on the globe (small islands magnified) (Polynesia centered).svg
, map_alt = Location of New Caledonia
, map_caption = Location of New Caledonia
, mapsize = 290px
, subdivision_type = Sovereign st ...
( France) (190,000 t), Australia
Australia, officially the Commonwealth of Australia, is a Sovereign state, sovereign country comprising the mainland of the Australia (continent), Australian continent, the island of Tasmania, and numerous List of islands of Australia, sma ...
(160,000 t) and Canada (130,000 t) are the largest producers as of 2021. The largest nickel deposits in non-Russian Europe are in Finland and Greece. Identified land-based sources averaging at least 1% nickel contain at least 130 million tonnes of nickel. About 60% is in laterites and 40% is in sulfide deposits. Also, extensive nickel sources are found in the depths of the Pacific Ocean, especially in an area called the Clarion Clipperton Zone
The Clipperton Fracture Zone, also known as the Clarion-Clipperton Zone, is a geological submarine fracture zone of the Pacific Ocean, with a length of around 4500 miles (7240 km). The zone spans approximately . It is one of the five major l ...
in the form of polymetallic nodules
Polymetallic nodules, also called manganese nodules, are mineral concretions on the sea bottom formed of concentric layers of iron and manganese hydroxides around a core. As nodules can be found in vast quantities, and contain valuable metals, dep ...
peppering the seafloor at 3.5–6 km below sea level. These nodules are composed of numerous rare-earth metals and are estimated to be 1.7% nickel. With advances in science and engineering, regulation is currently being set in place by the International Seabed Authority to ensure that these nodules are collected in an environmentally conscientious manner while adhering to the United Nations Sustainable Development Goals
The Sustainable Development Goals (SDGs) or Global Goals are a collection of 17 interlinked objectives designed to serve as a "shared blueprint for peace and prosperity for people and the planet, now and into the future".United Nations (2017) R ...
.
The one place in the United States where nickel has been profitably mined is Riddle, Oregon, with several square miles of nickel-bearing garnierite surface deposits. The mine closed in 1987. The Eagle mine project is a new nickel mine in Michigan's Upper Peninsula. Construction was completed in 2013, and operations began in the third quarter of 2014. In the first full year of operation, the Eagle Mine produced 18,000 t.
Production
stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corros ...
applications, 75% pure nickel can be used without further purification, depending on impurities.
Traditionally, most sulfide ores are processed using pyrometallurgical techniques to produce a matte for further refining. Recent advances in hydrometallurgical techniques result in significantly purer metallic nickel product. Most sulfide deposits have traditionally been processed by concentration through a froth flotation process followed by pyrometallurgical extraction. In hydrometallurgical processes, nickel sulfide ores are concentrated with flotation (differential flotation if Ni/Fe ratio is too low) and then smelted. The nickel matte is further processed with the Sherritt-Gordon process. First, copper is removed by adding hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
, leaving a concentrate of cobalt and nickel. Then, solvent extraction is used to separate the cobalt and nickel, with the final nickel content greater than 99%.

Electrorefining
A second common refining process is leaching the metal matte into a nickel salt solution, followed byelectrowinning
Electrowinning, also called electroextraction, is the electrodeposition of metals from their ores that have been put in solution via a process commonly referred to as leaching. Electrorefining uses a similar process to remove impurities from a ...
the nickel from solution by plating it onto a cathode as electrolytic nickel.
Mond process
 The purest metal is obtained from nickel oxide by the Mond process, which gives a purity of over 99.99%. The process was patented by Ludwig Mond and has been in industrial use since before the beginning of the 20th century. In this process, nickel is reacted with carbon monoxide in the presence of a sulfur catalyst at around 40–80 °C to form nickel carbonyl. In a similar reaction with iron, iron pentacarbonyl can form, though this reaction is slow. If necessary, the nickel may be separated by distillation.
The purest metal is obtained from nickel oxide by the Mond process, which gives a purity of over 99.99%. The process was patented by Ludwig Mond and has been in industrial use since before the beginning of the 20th century. In this process, nickel is reacted with carbon monoxide in the presence of a sulfur catalyst at around 40–80 °C to form nickel carbonyl. In a similar reaction with iron, iron pentacarbonyl can form, though this reaction is slow. If necessary, the nickel may be separated by distillation. Dicobalt octacarbonyl
Dicobalt octacarbonyl is an organocobalt compound with composition . This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent me ...
is also formed in nickel distillation as a by-product, but it decomposes to tetracobalt dodecacarbonyl at the reaction temperature to give a non-volatile solid.
Nickel is obtained from nickel carbonyl by one of two processes. It may be passed through a large chamber at high temperatures in which tens of thousands of nickel spheres (pellets) are constantly stirred. The carbonyl decomposes and deposits pure nickel onto the spheres. In the alternate process, nickel carbonyl is decomposed in a smaller chamber at 230 °C to create a fine nickel powder. The byproduct carbon monoxide is recirculated and reused. The highly pure nickel product is known as "carbonyl nickel".
Market value
The market price of nickel surged throughout 2006 and the early months of 2007; , the metal was trading at US$52,300/ tonne or $1.47/oz. The price later fell dramatically; , the metal was trading at $11,000/tonne, or $0.31/oz. During the2022 Russian invasion of Ukraine
On 24 February 2022, in a major escalation of the Russo-Ukrainian War, which began in 2014. The invasion has resulted in tens of thousands of deaths on both sides. It has caused Europe's largest refugee crisis since World War II. An ...
, worries about sanctions on Russian nickel exports triggered a short squeeze, causing the price of nickel to quadruple in just two days, reaching US$100,000 per tonne. The London Metal Exchange cancelled contracts worth $3.9 billion and suspended nickel trading for over a week. Analyst Andy Home argued that such price shocks are exacerbated by the purity requirements imposed by metal markets: only Grade I (99.8% pure) metal can be used as a commodity
In economics, a commodity is an economic good, usually a resource, that has full or substantial fungibility: that is, the market treats instances of the good as equivalent or nearly so with no regard to who produced them.
The price of a comm ...
on the exchanges, but most of the world's supply is either in ferro-nickel alloys or lower-grade purities.
Applications
 Global use of nickel is currently 68% in stainless steel, 10% in nonferrous alloys, 9%
Global use of nickel is currently 68% in stainless steel, 10% in nonferrous alloys, 9% electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be ...
, 7% alloy steel, 3% foundries, and 4% other (including batteries).
Nickel is used in many recognizable industrial and consumer products, including stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corros ...
, alnico magnets, coinage, rechargeable batteries (e.g. nickel-iron), electric guitar strings, microphone capsules, plating on plumbing fixtures, and special alloys such as permalloy
Permalloy is a nickel–iron magnetic alloy, with about 80% nickel and 20% iron content. Invented in 1914 by physicist Gustav Elmen at Bell Telephone Laboratories, it is notable for its very high magnetic permeability, which makes it useful as a ...
, elinvar, and invar. It is used for plating and as a green tint in glass. Nickel is preeminently an alloy metal, and its chief use is in nickel steels and nickel cast irons, in which it typically increases the tensile strength, toughness, and elastic limit. It is widely used in many other alloys, including nickel brasses and bronzes and alloys with copper, chromium, aluminium, lead, cobalt, silver, and gold ( Inconel, Incoloy, Monel, Nimonic).
 Because nickel is resistant to corrosion, it was occasionally used as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above), but in the later years of the 20th century, it was replaced by cheaper
Because nickel is resistant to corrosion, it was occasionally used as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above), but in the later years of the 20th century, it was replaced by cheaper stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corros ...
(i.e., iron) alloys, except in the United States and Canada.
Nickel is an excellent alloying agent for certain precious metals and is used in the fire assay as a collector of platinum group elements (PGE). As such, nickel can fully collect all six PGEs from ores, and can partially collect gold. High-throughput nickel mines may also do PGE recovery (mainly platinum and palladium); examples are Norilsk, Russia and the Sudbury Basin, Canada.
Nickel foam or nickel mesh is used in gas diffusion electrodes for alkaline fuel cells.
Nickel and its alloys are often used as catalysts for hydrogenation reactions. Raney nickel, a finely divided nickel-aluminium alloy, is one common form, though related catalysts are also used, including Raney-type catalysts.
Nickel is naturally magnetostrictive: in the presence of a magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
, the material undergoes a small change in length. The magnetostriction of nickel is on the order of 50 ppm and is negative, indicating that it contracts.
Nickel is used as a binder in the cemented tungsten carbide or hardmetal industry and used in proportions of 6% to 12% by weight. Nickel makes the tungsten carbide magnetic and adds corrosion-resistance to the cemented parts, though the hardness is less than those with cobalt binder.
, with half-life 100.1 years, is useful in krytron devices as a beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β� ...
(high-speed electron) emitter to make ionization by the keep-alive electrode more reliable. It is being investigated as a power source for betavoltaic batteries.
Around 27% of all nickel production is used for engineering, 10% for building and construction, 14% for tubular products, 20% for metal goods, 14% for transport, 11% for electronic goods, and 5% for other uses.
Raney nickel is widely used for hydrogenation of unsaturated oils to make margarine
Margarine (, also , ) is a spread used for flavoring, baking, and cooking. It is most often used as a substitute for butter. Although originally made from animal fats, most margarine consumed today is made from vegetable oil. The spread was orig ...
, and substandard margarine and leftover oil may contain nickel as a contaminant. Forte et al. found that type 2 diabetic patients have 0.89 ng/mL of Ni in the blood relative to 0.77 ng/mL in control subjects.
Biological role
It was not recognized until the 1970s, but nickel is known to play an important role in the biology of some plants, bacteria,archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
, and fungi. Nickel enzymes such as urease are considered virulence factors in some organisms. Urease catalyzes hydrolysis of urea to form ammonia and carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally o ...
. NiFe hydrogenases can catalyze oxidation of to form protons and electrons; and also the reverse reaction, the reduction of protons to form hydrogen gas. A nickel-tetrapyrrole coenzyme, cofactor F430, is present in methyl coenzyme M reductase, which can catalyze the formation of methane, or the reverse reaction, in methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens are com ...
ic archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
(in +1 oxidation state). One of the carbon monoxide dehydrogenase enzymes consists of an Fe-Ni- S cluster. Other nickel-bearing enzymes include a rare bacterial class of superoxide dismutase
Superoxide dismutase (SOD, ) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide () radical into ordinary molecular oxygen (O2) and hydrogen peroxide (). Superoxide is produced as a by-product of oxygen me ...
and glyoxalase I enzymes in bacteria and several eukaryotic trypanosomal parasites (in other organisms, including yeast and mammals, this enzyme contains divalent ).
Dietary nickel may affect human health through infections by nickel-dependent bacteria, but nickel may also be an essential nutrient for bacteria living in the large intestine, in effect functioning as a prebiotic. The US Institute of Medicine has not confirmed that nickel is an essential nutrient for humans, so neither a Recommended Dietary Allowance (RDA) nor an Adequate Intake have been established. The tolerable upper intake level of dietary nickel is 1 mg/day as soluble nickel salts. Estimated dietary intake is 70 to 100 µg/day; less than 10% is absorbed. What is absorbed is excreted in urine. Relatively large amounts of nickel – comparable to the estimated average ingestion above – leach into food cooked in stainless steel. For example, the amount of nickel leached after 10 cooking cycles into one serving of tomato sauce averages 88 µg.
Nickel released from Siberian Traps volcanic eruptions is suspected of helping the growth of '' Methanosarcina'', a genus of euryarchaeote archaea that produced methane in the Permian–Triassic extinction event, the biggest known mass extinction.
Toxicity
The major source of nickel exposure is oral consumption, as nickel is essential to plants. Typical background concentrations of nickel do not exceed 20 ng/m in air, 100 mg/kg in soil, 10 mg/kg in vegetation, 10 μg/L in freshwater and 1 μg/L in seawater. Environmental concentrations may be increased by human pollution. For example, nickel-plated faucets may contaminate water and soil; mining and smelting may dump nickel into wastewater; nickel–steel alloy cookware and nickel-pigmented dishes may release nickel into food. Air may be polluted by nickel ore refining andfossil fuel
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels m ...
combustion. Humans may absorb nickel directly from tobacco smoke and skin contact with jewelry, shampoos, detergents, and coins. A less common form of chronic exposure is through hemodialysis as traces of nickel ions may be absorbed into the plasma from the chelating action of albumin.
The average daily exposure is not a threat to human health. Most nickel absorbed by humans is removed by the kidneys and passed out of the body through urine or is eliminated through the gastrointestinal tract without being absorbed. Nickel is not a cumulative poison, but larger doses or chronic inhalation exposure may be toxic, even carcinogenic, and constitute an occupational hazard.
Nickel compounds are classified as human carcinogensIARC (2012)"Nickel and nickel compounds"
in ''IARC Monogr Eval Carcinog Risks Hum''. Volume 100C. pp. 169–218..Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006 J L 353, 31.12.2008, p. 1br>Annex VI
. Accessed July 13, 2017.Globally Harmonised System of Classification and Labelling of Chemicals (GHS)
, 5th ed., United Nations, New York and Geneva, 2013..National Toxicology Program. (2016)
, 14th ed. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service.. based on increased respiratory cancer risks observed in epidemiological studies of sulfidic ore refinery workers. This is supported by the positive results of the NTP bioassays with Ni sub-sulfide and Ni oxide in rats and mice. The human and animal data consistently indicate a lack of carcinogenicity via the oral route of exposure and limit the carcinogenicity of nickel compounds to respiratory tumours after inhalation. Nickel metal is classified as a suspect carcinogen; there is consistency between the absence of increased respiratory cancer risks in workers predominantly exposed to metallic nickel and the lack of respiratory tumours in a rat lifetime inhalation carcinogenicity study with nickel metal powder. In the rodent inhalation studies with various nickel compounds and nickel metal, increased lung inflammations with and without bronchial lymph node hyperplasia or fibrosis were observed. In rat studies, oral ingestion of water-soluble nickel salts can trigger perinatal mortality in pregnant animals. Whether these effects are relevant to humans is unclear as epidemiological studies of highly exposed female workers have not shown adverse developmental toxicity effects. People can be exposed to nickel in the workplace by inhalation, ingestion, and contact with skin or eye. The Occupational Safety and Health Administration (OSHA) has set the legal limit ( permissible exposure limit) for the workplace at 1 mg/m per 8-hour workday, excluding nickel carbonyl. The National Institute for Occupational Safety and Health (NIOSH) sets the recommended exposure limit (REL) at 0.015 mg/m per 8-hour workday. At 10 mg/m, nickel is
immediately dangerous to life and health
The term immediately dangerous to life or health (IDLH) is defined by the US National Institute for Occupational Safety and Health (NIOSH) as exposure to airborne contaminants that is "likely to cause death or immediate or delayed permanent advers ...
. Nickel carbonyl is an extremely toxic gas. The toxicity of metal carbonyls is a function of both the toxicity of the metal and the off-gassing of carbon monoxide from the carbonyl functional groups; nickel carbonyl is also explosive in air.
Sensitized persons may show a skin contact allergy to nickel known as a contact dermatitis. Highly sensitized persons may also react to foods with high nickel content. Patients with pompholyx may also be sensitive to nickel. Nickel is the top confirmed contact allergen worldwide, partly due to its use in jewelry for pierced ear
An earring is a piece of jewelry attached to the ear via a piercing in the earlobe or another external part of the ear (except in the case of clip earrings, which clip onto the lobe). Earrings have been worn by people in different civilizations an ...
s. Nickel allergies affecting pierced ears are often marked by itchy, red skin. Many earrings are now made without nickel or with low-release nickel to address this problem. The amount allowed in products that contact human skin is now regulated by the European Union. In 2002, researchers found that the nickel released by 1 and 2 euro coins, far exceeded those standards. This is believed to be due to a galvanic reaction. Nickel was voted Allergen of the Year Allergen of the Year is an annual award voted upon by the American Contact Dermatitis Society. This is "designed to draw attention to allergens that are very common, under-recognized, merit more attention because they are causing significant allergi ...
in 2008 by the American Contact Dermatitis Society. In August 2015, the American Academy of Dermatology
The American Academy of Dermatology (AAD) is a non-profit professional organization of dermatologists in the United States and Canada, based in Rosemont, Illinois, near Chicago. It was founded in 1938 and has more than 20,500 members. The Academy ...
adopted a position statement on the safety of nickel: "Estimates suggest that contact dermatitis, which includes nickel sensitization, accounts for approximately $1.918 billion and affects nearly 72.29 million people."Position Statement on Nickel Sensitivity. American Academy of Dermatology(August 22, 2015) Reports show that both the nickel-induced activation of hypoxia-inducible factor (HIF-1) and the up-regulation of hypoxia-inducible genes are caused by depletion of intracellular
ascorbate
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) and ...
. The addition of ascorbate to the culture medium increased the intracellular ascorbate level and reversed both the metal-induced stabilization of HIF-1- and HIF-1α-dependent gene expression.
References
External links
Nickel
at '' The Periodic Table of Videos'' (University of Nottingham)
CDC – Nickel – NIOSH Workplace Safety and Health Topic
An occupational hygiene assessment of dermal nickel exposures in primary production industries
by GW Hughson. Institute of Occupational Medicine Research Report TM/04/05
An occupational hygiene assessment of dermal nickel exposures in primary production and primary user industries. Phase 2 Report
by GW Hughson. Institute of Occupational Medicine Research Report TM/05/06
"The metal that brought you cheap flights"
BBC News {{Authority control Chemical elements Transition metals Dietary minerals Ferromagnetic materials IARC Group 2B carcinogens Native element minerals Chemical elements with face-centered cubic structure