Metal peroxide on:
[Wikipedia]
[Google]
[Amazon]
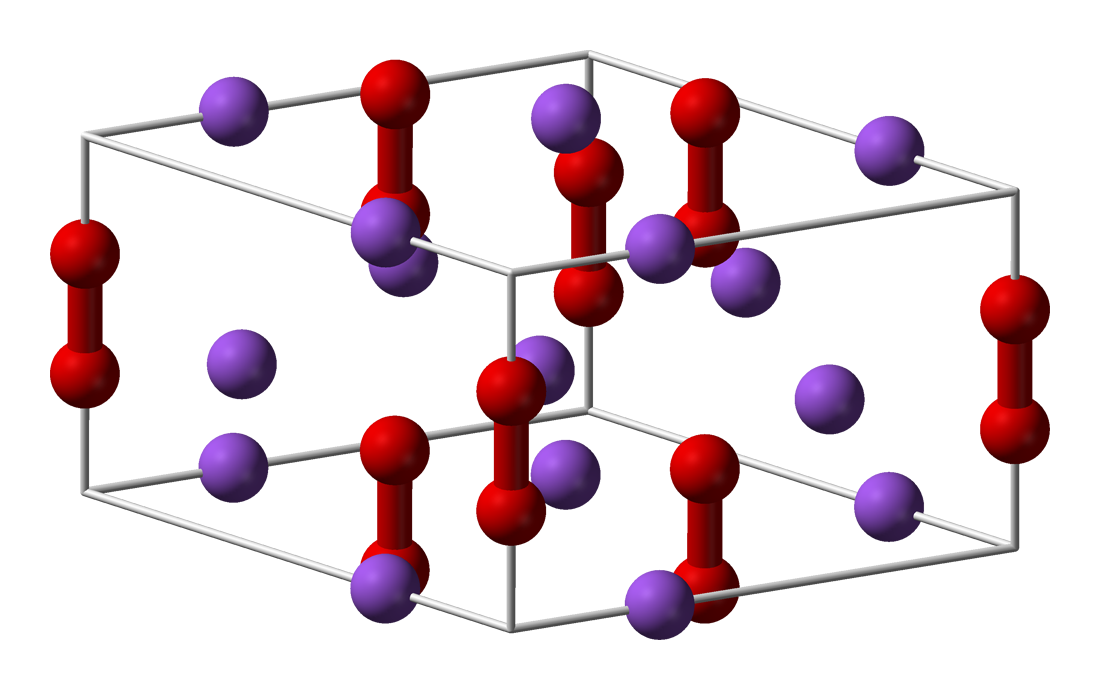 Metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide () groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid (H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of
Metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide () groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid (H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of
 The peroxide ion is composed of two oxygen atoms that are linked by a single bond. The molecular orbital diagram of the peroxide dianion predicts a doubly occupied antibonding π* orbital and a bond order of 1. The bond length is 149 pm, which is larger than in the ground state ( triplet oxygen) of the oxygen molecule (3O2, 121 pm). This translates into the smaller force constant of the bond (2.8 N/cm vs. 11.4 N/cm for 3O2) and the lower
The peroxide ion is composed of two oxygen atoms that are linked by a single bond. The molecular orbital diagram of the peroxide dianion predicts a doubly occupied antibonding π* orbital and a bond order of 1. The bond length is 149 pm, which is larger than in the ground state ( triplet oxygen) of the oxygen molecule (3O2, 121 pm). This translates into the smaller force constant of the bond (2.8 N/cm vs. 11.4 N/cm for 3O2) and the lower
''Inorganic Chemistry''
Academic Press, 2001, , pp. 475 ff The peroxide ion can be compared with superoxide , which is a radical, and dioxygen, a diradical.
''Inorganic Chemistry''
Academic Press, 2001, , pp. 471–502 : Barium peroxide was once used to produce pure oxygen from air. This process relies on the temperature-dependent chemical balance between barium oxide and peroxide: the reaction of barium oxide with air at 500 °C results in barium peroxide, which upon heating to above 700 °C in oxygen decomposes back to barium oxide releasing pure oxygen. The lighter alkaline earth metals

 Metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide () groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid (H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of
Metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide () groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid (H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s have a more covalent character.
Bonding in O22−
 The peroxide ion is composed of two oxygen atoms that are linked by a single bond. The molecular orbital diagram of the peroxide dianion predicts a doubly occupied antibonding π* orbital and a bond order of 1. The bond length is 149 pm, which is larger than in the ground state ( triplet oxygen) of the oxygen molecule (3O2, 121 pm). This translates into the smaller force constant of the bond (2.8 N/cm vs. 11.4 N/cm for 3O2) and the lower
The peroxide ion is composed of two oxygen atoms that are linked by a single bond. The molecular orbital diagram of the peroxide dianion predicts a doubly occupied antibonding π* orbital and a bond order of 1. The bond length is 149 pm, which is larger than in the ground state ( triplet oxygen) of the oxygen molecule (3O2, 121 pm). This translates into the smaller force constant of the bond (2.8 N/cm vs. 11.4 N/cm for 3O2) and the lower frequency
Frequency is the number of occurrences of a repeating event per unit of time. It is also occasionally referred to as ''temporal frequency'' for clarity, and is distinct from ''angular frequency''. Frequency is measured in hertz (Hz) which is eq ...
of the molecular vibration (770 cm−1 vs. 1555 cm−1 for 3O2).Wiberg, Egon; Wiberg, Nils and Holleman, Arnold Frederic''Inorganic Chemistry''
Academic Press, 2001, , pp. 475 ff The peroxide ion can be compared with superoxide , which is a radical, and dioxygen, a diradical.
Preparation of peroxide salts
Most alkali metal peroxides can be synthesized directly by oxygenation of the elements. Lithium peroxide is formed upon treating lithium hydroxide with hydrogen peroxide:Vol'nov, I. I. ''Peroxides, superoxides and ozonides of alkali and alkaline earth metals'', pp. 21–51, Plenum Press, New York, 1966, no ISBN : 2 LiOH + H2O2 → Li2O2 + 2 H2O Barium peroxide is prepared by oxygenation of barium oxide at elevated temperature and pressure.Wiberg, Egon; Wiberg, Nils and Holleman, Arnold Frederic''Inorganic Chemistry''
Academic Press, 2001, , pp. 471–502 : Barium peroxide was once used to produce pure oxygen from air. This process relies on the temperature-dependent chemical balance between barium oxide and peroxide: the reaction of barium oxide with air at 500 °C results in barium peroxide, which upon heating to above 700 °C in oxygen decomposes back to barium oxide releasing pure oxygen. The lighter alkaline earth metals
calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
and strontium also form peroxides, which are used commercially as oxygen sources or oxidizers.
Reaction of peroxide salts
Few reactions are generally formulated for peroxide salt. In excess of dilute acids or water they release hydrogen peroxide. :Na2O2 + 2 HCl → 2 NaCl + H2O2 Upon heating, the reaction with water leads to the release of oxygen. Upon exposure to air, alkali metal peroxides absorb CO2 to give peroxycarbonates.Transition metal peroxides
Unlike the alkali metal and alkaline earth metal peroxides, binary transition metal peroxides, that is, compounds containing only metal cations and peroxide anions, are rare. Metal dioxides are pervasive, such as MnO2 and rutile (TiO2), but these are oxides, not peroxides. Well characterized examples include the d10 metal cations: zinc peroxide (ZnO2), two polymorphs (both explosive) ofmercury peroxide
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
(HgO2), and cadmium peroxide (CdO2).
Peroxide is a common ligand in metal complexes. Within the area of transition metal dioxygen complex
Transition or transitional may refer to:
Mathematics, science, and technology Biology
* Transition (genetics), a point mutation that changes a purine nucleotide to another purine (A ↔ G) or a pyrimidine nucleotide to another pyrimidine (C ↔ ...
es, functions as a bidentate ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
. Some complexes have only peroxide ligands, for example, chromium(VI) oxide peroxide
Chromium(VI) peroxide or chromium oxide peroxide is an unstable compound with the formula CrO5. This compound contains one oxo ligand and two peroxo ligands, making a total of five oxygen atoms per chromium atom.
Preparation and properties
Chromi ...
(). Similarly, molybdate
In chemistry a molybdate is a compound containing an oxoanion with molybdenum in its highest oxidation state of 6. Molybdenum can form a very large range of such oxoanions which can be discrete structures or polymeric extended structures, altho ...
reacts in alkaline media with peroxide to form red peroxomolybdate . The reaction of hydrogen peroxide with aqueous titanium(IV) gives a brightly colored peroxy complex that is a useful test for titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion i ...
as well as hydrogen peroxide. Many transition metal dioxygen complex
Transition or transitional may refer to:
Mathematics, science, and technology Biology
* Transition (genetics), a point mutation that changes a purine nucleotide to another purine (A ↔ G) or a pyrimidine nucleotide to another pyrimidine (C ↔ ...
es are best described as adducts of peroxide.
Applications
Many inorganic peroxides are used for bleaching textiles andpaper
Paper is a thin sheet material produced by mechanically or chemically processing cellulose fibres derived from wood, rags, grasses or other vegetable sources in water, draining the water through fine mesh leaving the fibre evenly distribu ...
and as a bleaching additive to detergents and cleaning products. The increasing environmental concerns resulted in the preference of peroxides over chlorine-based compounds and a sharp increase in the peroxide production.
The past use of perborates as additives to detergents and cleaning productsBrotherton, B.J. "Boron: Inorganic Chemistry", in ''Encyclopedia of Inorganic Chemistry'' (1994) Ed. R. Bruce King, John Wiley & Sons has been largely replaced by percarbonates. The use of peroxide compounds in detergents is often reflected in their trade names; for example, Persil is a combination of the words ''per''borate and ''sil''icate.
Some peroxide salts release oxygen upon reaction with carbon dioxide. This reaction is used in generation of oxygen from exhaled carbon dioxide on submarine
A submarine (or sub) is a watercraft capable of independent operation underwater. It differs from a submersible, which has more limited underwater capability. The term is also sometimes used historically or colloquially to refer to remotely op ...
s and spaceships. Sodium or lithium peroxides are preferred in space applications because of their lower molar mass and therefore higher oxygen yield per unit weight.
: 2 Na2O2 + 2 CO2 → 2 Na2CO3 + O2
Alkali metal peroxides can be used for the synthesis of organic peroxides. One example is the conversion of benzoyl chloride with sodium peroxide to dibenzoyl peroxide
Benzoyl peroxide is a chemical compound (specifically, an organic peroxide) with structural formula , often abbreviated as (BzO)2. In terms of its structure, the molecule can be described as two benzoyl (, Bz) groups connected by a peroxide ( ...
.

History
Alexander von Humboldt
Friedrich Wilhelm Heinrich Alexander von Humboldt (14 September 17696 May 1859) was a German polymath, geographer, naturalist, explorer, and proponent of Romantic philosophy and science. He was the younger brother of the Prussian minister ...
synthesized barium peroxide in 1799 as a byproduct of his attempts to decompose air.
Nineteen years later Louis Jacques Thénard recognized that this compound could be used for the preparation of hydrogen peroxide.C. W. Jones, J. H. Clark. ''Applications of Hydrogen Peroxide and Derivatives''. Royal Society of Chemistry, 1999. Thénard and Joseph Louis Gay-Lussac synthesized sodium peroxide
Sodium peroxide is an inorganic compound with the formula Na2O2. This yellowish solid is the product of sodium ignited in excess oxygen. It is a strong base. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2O ...
in 1811. The bleaching effect of peroxides and their salts on natural dye
Natural dyes are dyes or colorants derived from plants, invertebrates, or minerals. The majority of natural dyes are vegetable dyes from plant sources— roots, berries, bark, leaves, and wood—and other biological sources such as fungi.
Ar ...
s became known around that time, but early attempts of industrial production of peroxides failed, and the first plant producing hydrogen peroxide was built in 1873 in Berlin
Berlin ( , ) is the capital and largest city of Germany by both area and population. Its 3.7 million inhabitants make it the European Union's most populous city, according to population within city limits. One of Germany's sixteen constitu ...
.
See also
* Ozonide, * Superoxide, *Hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
References
{{reflist, 35em Anions