|
Calcium Peroxide
Calcium peroxide or calcium dioxide is the inorganic compound with the formula CaO2. It is the peroxide (O22−) salt of Ca2+. Commercial samples can be yellowish, but the pure compound is white. It is almost insoluble in water. Structure and stability As a solid, it is relatively stable against decomposition. In contact with water however it hydrolyzes with release of oxygen. Upon treatment with an acid, it forms hydrogen peroxide. Preparation Calcium peroxide is produced by combining calcium salts and hydrogen peroxide: :Ca(OH)2 + H2O2 → CaO2 + 2 H2O The octahydrate precipitates upon the reaction of calcium hydroxide with dilute hydrogen peroxide. Upon heating it dehydrates. Applications It is mainly used as an oxidant to enhance the extraction of precious metals from their ores. In its second main application, it is used as a food additive under the E number E930 it is used as flour bleaching agent and improving agent. In agriculture it is used in the pres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, Caustic (substance), caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime (material), lime''" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime. Quicklime is relatively inexpensive. Both it and a chemical derivative (calcium hydroxide, of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flour Bleaching Agent
Flour bleaching agent is the agent added to fresh milled grains to whiten the flour by removing the yellow colour pigment called xanthophyll. It whitens the flour, which is used in the baking industry. Overview Usual flour bleaching agents are: *Organic peroxides (benzoyl peroxide) *Calcium peroxide *Chlorine *Chlorine dioxide *Azodicarbonamide *Nitrogen dioxide *Atmospheric oxygen, used during natural aging of flour Use of chlorine, bromates, and peroxides is not allowed in the European Union. Bleached flour improves the structure-forming capacity, allowing the use of dough formulas with lower proportions of flour and higher proportions of sugar . In biscuit making, use of chlorinated flour reduces the spread of the dough, and provides a "tighter" surface. The changes of functional properties of the flour proteins are likely to be caused by their oxidation. In countries where bleached flour is prohibited, microwaving plain flour produces similar chemical changes to the bleaching ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Additives
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), salt (salting), smoke (smoking), sugar (crystallization), etc. This allows for longer-lasting foods such as bacon, sweets or wines. With the advent of processed foods in the second half of the twentieth century, many additives have been introduced, of both natural and artificial origin. Food additives also include substances that may be introduced to food indirectly (called "indirect additives") in the manufacturing process, through packaging, or during storage or transport. Numbering To regulate these additives and inform consumers, each additive is assigned a unique number called an "E number", which is used in Europe for all approved additives. This numbering scheme has now been adopted and extended by the '' Codex Alimentarius'' Commission t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Compounds
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxides
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide (). * Organic peroxide In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
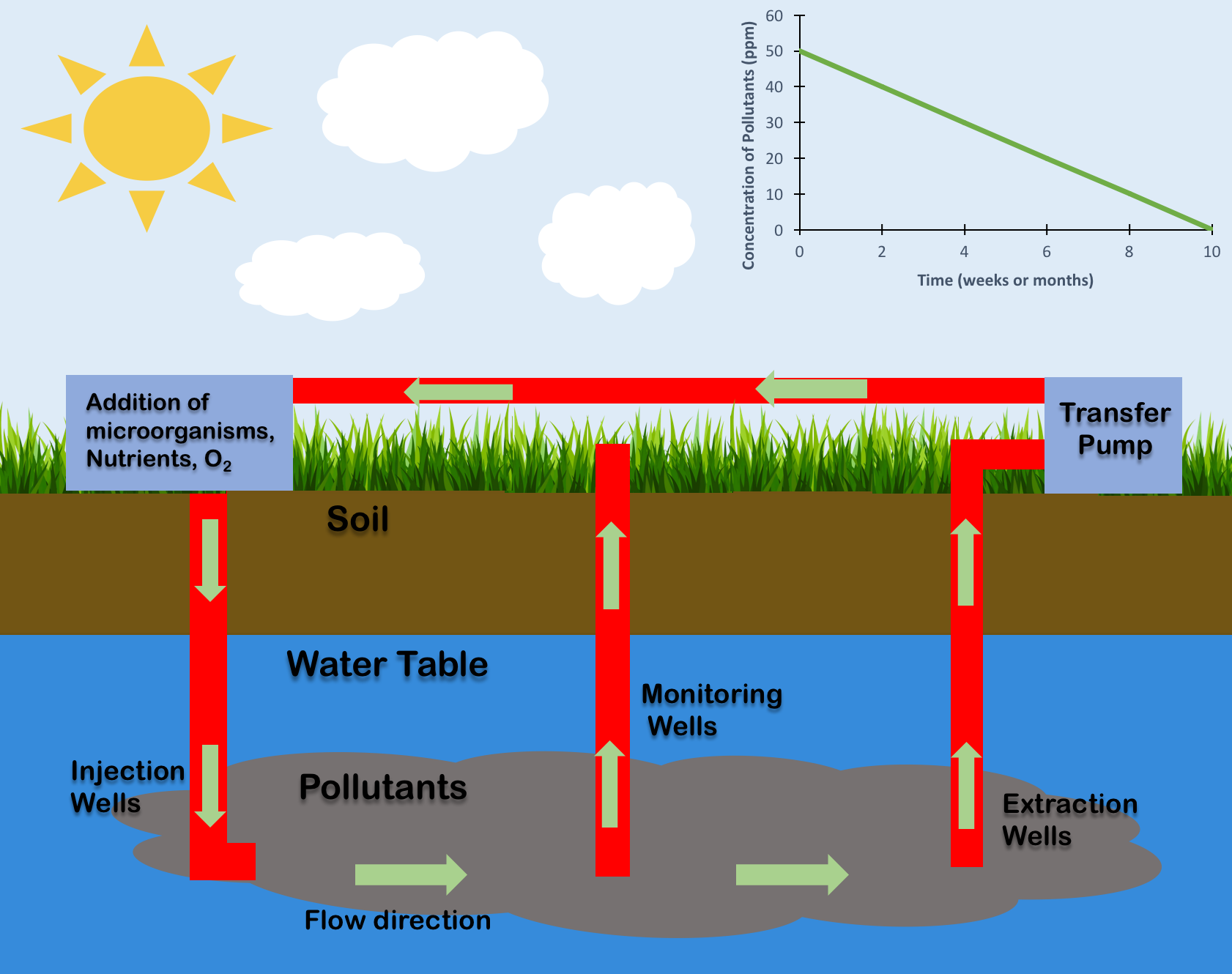
Bioremediation
Bioremediation broadly refers to any process wherein a biological system (typically bacteria, microalgae, fungi, and plants), living or dead, is employed for removing environmental pollutants from air, water, soil, flue gasses, industrial effluents etc., in natural or artificial settings. The natural ability of organisms to adsorb, accumulate, and degrade common and emerging pollutants has attracted the use of biological resources in treatment of contaminated environment. In comparison to conventional physicochemical treatment methods bioremediation may offer considerable advantages as it aims to be sustainable, eco-friendly, cheap, and scalable. Most bioremediation is inadvertent, involving native organisms. Research on bioremediation is heavily focused on stimulating the process by inoculation of a polluted site with organisms or supplying nutrients to promote the growth. In principle, bioremediation could be used to reduce the impact of byproducts created from anthropogenic acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Peroxide
Magnesium peroxide (MgO2) is an odorless fine powder peroxide with a white to off-white color. It is similar to calcium peroxide because magnesium peroxide also releases oxygen by breaking down at a controlled rate with water. Commercially, magnesium peroxide often exists as a compound of magnesium peroxide and magnesium hydroxide. Structure O2, similarly to N2, has the ability to bind either side-on or end-on. The structure of MgO2 has been calculated as a triangular shape with the O2 molecule binding side-on to the magnesium. This arrangement is a result of the Mg+ donating charge to the oxygen and creating a Mg2+O22−. The bond between to O2 and the magnesium atom has an approximate dissociation energy of 90 kJ mol−1. In the solid state, MgO2 has a cubic pyrite-type crystal structure with 6-coordinate Mg2+ ions and O22− peroxide-groups, according to experimental data and evolutionary crystal structure prediction, the latter predicting a phase transition at the pressure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aquaculture
Aquaculture (less commonly spelled aquiculture), also known as aquafarming, is the controlled cultivation ("farming") of aquatic organisms such as fish, crustaceans, mollusks, algae and other organisms of value such as aquatic plants (e.g. lotus). Aquaculture involves cultivating freshwater, brackish water and saltwater populations under controlled or semi-natural conditions, and can be contrasted with commercial fishing, which is the harvesting of wild fish. Mariculture, commonly known as marine farming, refers specifically to aquaculture practiced in seawater habitats and lagoons, opposed to in freshwater aquaculture. Pisciculture is a type of aquaculture that consists of fish farming to obtain fish products as food. Aquaculture can also be defined as the breeding, growing, and harvesting of fish and other aquatic plants, also known as farming in water. It is an environmental source of food and commercial product which help to improve healthier habitats and used to recon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rice Seed
Rice is the seed of the grass species ''Oryza sativa'' (Asian rice) or less commonly ''Oryza glaberrima'' (African rice). The name wild rice is usually used for species of the genera ''Zizania'' and '' Porteresia'', both wild and domesticated, although the term may also be used for primitive or uncultivated varieties of ''Oryza''. As a cereal grain, domesticated rice is the most widely consumed staple food for over half of the world's human population,Abstract, "Rice feeds more than half the world's population." especially in Asia and Africa. It is the agricultural commodity with the third-highest worldwide production, after sugarcane and maize. Since sizable portions of sugarcane and maize crops are used for purposes other than human consumption, rice is the most important food crop with regard to human nutrition and caloric intake, providing more than one-fifth of the calories consumed worldwide by humans. There are many varieties of rice and culinary preferences tend to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Improving Agent
A dough conditioner, flour treatment agent, improving agent or bread improver is any ingredient or chemical added to bread dough to strengthen its texture or otherwise improve it in some way. Dough conditioners may include enzymes, yeast nutrients, mineral salts, oxidants and reductants, bleaching agents and emulsifiers. They are food additives combined with flour to improve baking functionality. Flour treatment agents are used to increase the speed of dough rising and to improve the strength and workability of the dough. While they are an important component of modern factory baking, some small-scale bakers reject them in favour of longer fermentation periods that produce greater depth of flavour. These agents are often sold as mixtures in a soy flour base, as only small amounts are required. Examples Examples of dough conditioners include ascorbic acid, distilled monoglycerides, citrate ester of monoglycerides, diglycerides, ammonium chloride, enzymes, diacetyl tartaric acid e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E Number
E numbers ("E" stands for "Europe") are codes for substances used as food additives, including those found naturally in many foods such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly found on food labels, their safety assessment and approval are the responsibility of the European Food Safety Authority (EFSA). The fact that an additive has an E number implies that its use was at one time permitted in products for sale in the European Single Market; some of these additives are no longer allowed today. Having a single unified list for food additives was first agreed upon in 1962 with food colouring. In 1964, the directives for preservatives were added, in 1970 antioxidants were added, in 1974 emulsifiers, stabilisers, thickeners and gelling agents were added as well. Numbering schemes The numbering scheme follows that of the International Numbering System (INS) as determined by the '' Codex Alimentarius'' committee, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |