Mercury (element) on:
[Wikipedia]
[Google]
[Amazon]
Mercury is a
 Mercury is a heavy, silvery-white metal that is liquid at room temperature. Compared to other metals, it is a poor conductor of heat, but a fair conductor of electricity. in
It has a
Mercury is a heavy, silvery-white metal that is liquid at room temperature. Compared to other metals, it is a poor conductor of heat, but a fair conductor of electricity. in
It has a
 Mercury dissolves many metals such as
Mercury dissolves many metals such as
 "Hg" is the modern
"Hg" is the modern
 Former mines in Italy, the United States and Mexico, which once produced a large proportion of the world supply, have now been completely mined out or, in the case of Slovenia (
Former mines in Italy, the United States and Mexico, which once produced a large proportion of the world supply, have now been completely mined out or, in the case of Slovenia (
 Mercury is used primarily for the manufacture of industrial chemicals or for electrical and electronic applications. It is used in some liquid-in-glass thermometers, especially those used to measure high temperatures. A still increasing amount is used as gaseous mercury in
Mercury is used primarily for the manufacture of industrial chemicals or for electrical and electronic applications. It is used in some liquid-in-glass thermometers, especially those used to measure high temperatures. A still increasing amount is used as gaseous mercury in
 Mercury and its compounds have been used in medicine, although they are much less common today than they once were, now that the toxic effects of mercury and its compounds are more widely understood. An example of the early therapeutic application of mercury of was published in 1787 by
Mercury and its compounds have been used in medicine, although they are much less common today than they once were, now that the toxic effects of mercury and its compounds are more widely understood. An example of the early therapeutic application of mercury of was published in 1787 by
File:Germicidal UV discharge tube glow rotate.jpg, The deep violet glow of a mercury vapor discharge in a
The Deep Space Atomic Clock (DSAC) under development by the

 Many historic applications made use of the peculiar physical properties of mercury, especially as a dense liquid and a liquid metal:
* Quantities of liquid mercury ranging from have been recovered from elite
Many historic applications made use of the peculiar physical properties of mercury, especially as a dense liquid and a liquid metal:
* Quantities of liquid mercury ranging from have been recovered from elite
 Preindustrial deposition rates of mercury from the atmosphere may be about 4 ng /(1 L of ice deposit). Although that can be considered a natural level of exposure, regional or global sources have significant effects. Volcanic eruptions can increase the atmospheric source by 4–6 times.
Natural sources, such as
Preindustrial deposition rates of mercury from the atmosphere may be about 4 ng /(1 L of ice deposit). Although that can be considered a natural level of exposure, regional or global sources have significant effects. Volcanic eruptions can increase the atmospheric source by 4–6 times.
Natural sources, such as
 Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The
Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The
Chemistry in its element podcast
(MP3) from the Royal Society of Chemistry's Chemistry World
Mercury
at ''The Periodic Table of Videos'' (University of Nottingham)
Centers for Disease Control and Prevention – Mercury Topic
Stopping Pollution: Mercury
– Oceana
Natural Resources Defense Council (NRDC): Mercury Contamination in Fish guide
– Natural Resources Defense Council, NRDC
NLM Hazardous Substances Databank – Mercury
BBC – Earth News – Mercury "turns" wetland birds such as ibises homosexual
Changing Patterns in the Use, Recycling, and Material Substitution of Mercury in the United States
United States Geological Survey
Thermodynamical data on liquid mercury.
* {{DEFAULTSORT:Mercury Mercury (element), Chemical elements Coolants Endocrine disruptors Native element minerals Neurotoxins Nuclear reactor coolants Occupational safety and health Transition metals
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol Hg and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
80. It is also known as quicksilver and was formerly named hydrargyrum ( ) from the Greek words, ''hydor'' (water) and ''argyros'' (silver). A heavy
Heavy may refer to:
Measures
* Heavy (aeronautics), a term used by pilots and air traffic controllers to refer to aircraft capable of 300,000 lbs or more takeoff weight
* Heavy, a characterization of objects with substantial weight
* Heavy, ...
, silvery d-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
; the only other element that is liquid under these conditions is the halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simila ...
, though metals such as caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
, gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
, and rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
melt just above room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
.
Mercury occurs in deposits throughout the world mostly as cinnabar
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of Mercury sulfide, mercury(II) sulfide (HgS). It is the most common source ore for refining mercury (element), elemental mercury and ...
(mercuric sulfide
Mercury sulfide, or mercury(II) sulfide is a chemical compound composed of the chemical elements mercury and sulfur. It is represented by the chemical formula HgS. It is virtually insoluble in water.
Crystal structure
HgS is dimorphic with ...
). The red pigment vermilion
Vermilion (sometimes vermillion) is a color, color family, and pigment most often made, since ancient history, antiquity until the 19th century, from the powdered mineral cinnabar (a form of mercury sulfide, which is toxic) and its correspondi ...
is obtained by grinding
Grind is the cross-sectional shape of a blade.
Grind, grinds, or grinding may also refer to:
Grinding action
* Grinding (abrasive cutting), a method of crafting
* Grinding (dance), suggestive club dancing
* Grinding (video gaming), repetitive and ...
natural cinnabar or synthetic mercuric sulfide.
Mercury is used in thermometer
A thermometer is a device that temperature measurement, measures temperature or a temperature gradient (the degree of hotness or coldness of an object). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb of a merc ...
s, barometer
A barometer is a scientific instrument that is used to measure air pressure in a certain environment. Pressure tendency can forecast short term changes in the weather. Many measurements of air pressure are used within surface weather analysis ...
s, manometer
Pressure measurement is the measurement of an applied force by a fluid (liquid or gas) on a surface. Pressure is typically measured in units of force per unit of surface area. Many techniques have been developed for the measurement of pressur ...
s, sphygmomanometer
A sphygmomanometer ( ), a blood pressure monitor, or blood pressure gauge, is a device used to measure blood pressure, composed of an inflatable cuff to collapse and then release the artery under the cuff in a controlled manner, and a mercury (e ...
s, float valve
A ballcock (also balltap or float valve) is a mechanism or machine for filling water tanks, such as those found in flush toilets, while avoiding overflow and (in the event of low water pressure) backflow. The modern ballcock was invented by J ...
s, mercury switch
A mercury switch is an electrical switch that opens and closes a circuit when a small amount of the liquid metal mercury connects metal electrodes to close the circuit. There are several different basic designs (tilt, displacement, radial, etc. ...
es, mercury relay
A mercury relay (mercury displacement relay, mercury contactor) is a relay that uses mercury as the switching element.
They are used as high-current switches or contactors, where contact erosion from constant cycling would be a problem for conven ...
s, fluorescent lamp
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet lig ...
s and other devices, though concerns about the element's toxicity have led to mercury thermometers and sphygmomanometers being largely phased out in clinical environments in favor of alternatives such as alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
- or galinstan
Galinstan (R) is a brand name for a alloy composed of gallium, indium, and tin which melts at and is thus liquid at room temperature. However, it is not a eutectic alloy but a near eutectic alloy. In scientific literature, galinstan is also used ...
-filled glass thermometers and thermistor
A thermistor is a type of resistor whose resistance is strongly dependent on temperature, more so than in standard resistors. The word thermistor is a portmanteau of ''thermal'' and ''resistor''.
Thermistors are divided based on their conduction ...
- or infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
-based electronic instruments. Likewise, mechanical pressure gauges and electronic strain gauge sensors have replaced mercury sphygmomanometers.
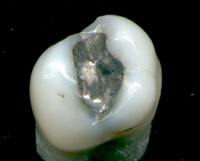
Mercury remains in use in scientific research applications and in amalgam
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital
...
for dental restoration
Dental restoration, dental fillings, or simply fillings are treatments used to restore the function, integrity, and morphology of missing tooth structure resulting from caries or external trauma as well as to the replacement of such structure sup ...
in some locales. It is also used in fluorescent lighting
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet ligh ...
. Electricity passed through mercury vapor in a fluorescent lamp produces short-wave ultraviolet light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
, which then causes the phosphor in the tube to fluoresce
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
, making visible light.
Mercury poisoning
Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type, dose, method, and duration of exposure. They may include muscle weakness, poor coordination, numbness in the hands and feet, skin rashe ...
can result from exposure to water-soluble forms of mercury (such as mercuric chloride
Mercury(II) chloride (or mercury bichloride, mercury dichloride), historically also known as sulema or corrosive sublimate, is the inorganic chemical compound of mercury and chlorine with the formula HgCl2. It is white crystalline solid and is a ...
or methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a ...
), by inhalation of mercury vapor, or by ingesting any form of mercury.
Properties
Physical properties
 Mercury is a heavy, silvery-white metal that is liquid at room temperature. Compared to other metals, it is a poor conductor of heat, but a fair conductor of electricity. in
It has a
Mercury is a heavy, silvery-white metal that is liquid at room temperature. Compared to other metals, it is a poor conductor of heat, but a fair conductor of electricity. in
It has a freezing point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends ...
of −38.83 °C and a boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
of 356.73 °C, both the lowest of any stable metal, although preliminary experiments on copernicium
Copernicium is a synthetic chemical element with the symbol Cn and atomic number 112. Its known isotopes are extremely radioactive, and have only been created in a laboratory. The most stable known isotope, copernicium-285, has a half-life of ap ...
and flerovium
Flerovium is a Transactinide element, superheavy chemical element with Chemical symbol, symbol Fl and atomic number 114. It is an extremely radioactive synthetic element. It is named after the Flerov Laboratory of Nuclear Reactions of the Joint ...
have indicated that they have even lower boiling points. This effect is due to lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radii ...
and relativistic contraction reducing the radius of the outermost electrons, and thus weakening the metallic bonding in mercury. Upon freezing, the volume of mercury decreases by 3.59% and its density changes from 13.69 g/cm3 when liquid to 14.184 g/cm3 when solid. The coefficient of volume expansion is 181.59 × 10−6 at 0 °C, 181.71 × 10−6 at 20 °C and 182.50 × 10−6 at 100 °C (per °C). Solid mercury is malleable and ductile and can be cut with a knife.
Table of thermal and physical properties of liquid mercury:
Chemical properties
Mercury does not react with most acids, such as dilutesulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
, although oxidizing acid
An oxidizing acid is a Brønsted acid that is a strong oxidizing agent. Most Brønsted acids can act as oxidizing agents, because the acidic proton can be reduced to hydrogen gas. Some acids contain other structures that act as stronger oxidizing ...
s such as concentrated sulfuric acid and nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
or aqua regia dissolve it to give sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ar ...
, nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
, and chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
. Like silver, mercury reacts with atmospheric hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
. Mercury reacts with solid sulfur flakes, which are used in mercury spill kits to absorb mercury (spill kits also use activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
and powdered zinc).
Amalgams
 Mercury dissolves many metals such as
Mercury dissolves many metals such as gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
and silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
to form amalgams
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital, an in ...
. Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
is an exception, and iron flasks have traditionally been used to trade mercury. Several other first row transition metals with the exception of manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
and zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
are also resistant in forming amalgams. Other elements that do not readily form amalgams with mercury include platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
. Sodium amalgam
Sodium amalgam, commonly denoted Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. Sodium am ...
is a common reducing agent in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, and is also used in high-pressure sodium
A sodium-vapor lamp is a gas-discharge lamp that uses sodium in an excited state to produce light at a characteristic wavelength near 589 nm.
Two varieties of such lamps exist: low pressure and high pressure. Low-pressure sodium lamps are ...
lamps.
Mercury readily combines with aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
to form a mercury-aluminium amalgam when the two pure metals come into contact. Since the amalgam destroys the aluminium oxide layer which protects metallic aluminium from oxidizing in-depth (as in iron rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH ...
ing), even small amounts of mercury can seriously corrode aluminium. For this reason, mercury is not allowed aboard an aircraft under most circumstances because of the risk of it forming an amalgam with exposed aluminium parts in the aircraft.
Mercury embrittlement is the most common type of liquid metal embrittlement.
Isotopes
There are seven stableisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
s of mercury, with being the most abundant (29.86%). The longest-lived radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s are with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
of 444 years, and with a half-life of 46.612 days. Most of the remaining radioisotopes have half-lives that are less than a day. and are the most often studied NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
-active nuclei, having spins of and respectively. For the synthesis of precious metals
The synthesis of precious metals involves the use of either nuclear reactors or particle accelerators to produce these elements.
Precious metals occurring as fission products
Ruthenium, rhodium
Ruthenium and rhodium are precious metals produce ...
two stable mercury isotopes are of potential interest - the trace isotope and the more abundant . Both are "one neutron removed" from , a radioisotope which decays to , the only known stable isotope of gold. However, the rarity of and the high energy requirements of nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
s "knocking out" a neutron from (either via photodisintegration
Photodisintegration (also called phototransmutation, or a photonuclear reaction) is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The ...
or via a (n,2n) reaction involving fast neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with ...
s), have thus far ruled out practical application of this "real philosopher's stone".
Etymology
chemical symbol
Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with th ...
for mercury. It is an abbreviation of , a romanized
Romanization or romanisation, in linguistics, is the conversion of text from a different writing system to the Roman (Latin) script, or a system for doing so. Methods of romanization include transliteration, for representing written text, and ...
form of the ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic peri ...
name for mercury, (). is a Greek compound word meaning "water-silver", from - (-), the root of () "water", and () "silver". Like the English name quicksilver ("living-silver"), this name was due to mercury's liquid and shiny properties.
The modern English name "mercury" comes from the planet Mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
. In medieval alchemy
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
, the seven known metals—quicksilver, gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
, silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
, lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
, and tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
—were associated with the seven planets. Quicksilver was associated with the fastest planet, which had been named after the Roman god Mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, who was associated with speed and mobility. The astrological symbol for the planet became one of the alchemical symbol
Alchemical symbols, originally devised as part of alchemy, were used to denote some elements and some compounds until the 18th century. Although notation like this was mostly standardized, style and symbol varied between alchemists, so this pag ...
s for the metal, and "Mercury" became an alternative name for the metal. Mercury is the only metal for which the alchemical planetary name survives, as it was decided it was preferable to "quicksilver" as a chemical name.
History
Mercury was found inEgyptian
Egyptian describes something of, from, or related to Egypt.
Egyptian or Egyptians may refer to:
Nations and ethnic groups
* Egyptians, a national group in North Africa
** Egyptian culture, a complex and stable culture with thousands of years of ...
tombs that date from 1500 BC.
In China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's most populous country, with a population exceeding 1.4 billion, slightly ahead of India. China spans the equivalent of five time zones and ...
and Tibet
Tibet (; ''Böd''; ) is a region in East Asia, covering much of the Tibetan Plateau and spanning about . It is the traditional homeland of the Tibetan people. Also resident on the plateau are some other ethnic groups such as Monpa people, ...
, mercury use was thought to prolong life, heal fractures, and maintain generally good health, although it is now known that exposure to mercury vapor leads to serious adverse health effects. The first emperor of a unified China, Qín Shǐ Huáng Dì—allegedly buried in a tomb
A tomb ( grc-gre, τύμβος ''tumbos'') is a :wikt:repository, repository for the remains of the dead. It is generally any structurally enclosed interment space or burial chamber, of varying sizes. Placing a corpse into a tomb can be ...
that contained rivers of flowing mercury on a model of the land he ruled, representative of the rivers of China—was reportedly killed by drinking a mercury and powdered jade
Jade is a mineral used as jewellery or for ornaments. It is typically green, although may be yellow or white. Jade can refer to either of two different silicate minerals: nephrite (a silicate of calcium and magnesium in the amphibole group of ...
mixture formulated by Qin Qin may refer to:
Dynasties and states
* Qin (state) (秦), a major state during the Zhou Dynasty of ancient China
* Qin dynasty (秦), founded by the Qin state in 221 BC and ended in 206 BC
* Daqin (大秦), ancient Chinese name for the Roman Emp ...
alchemists intended as an elixir of immortality. Khumarawayh ibn Ahmad ibn Tulun
Abu 'l-Jaysh Khumārawayh ibn Aḥmad ibn Ṭūlūn ( ar, أبو الجيش خمارويه بن أحمد بن طولون; 864 – 18 January 896) was a son of the founder of the Tulunid dynasty, Ahmad ibn Tulun. His father, the autonomous ruler ...
, the second Tulunid
The Tulunids (), were a Mamluk dynasty of Turkic origin who were the first independent dynasty to rule Egypt, as well as much of Syria, since the Ptolemaic dynasty. They were independent from 868, when they broke away from the central authority ...
ruler of Egypt (r. 884–896), known for his extravagance and profligacy, reportedly built a basin filled with mercury, on which he would lie on top of air-filled cushions and be rocked to sleep.
In November 2014 "large quantities" of mercury were discovered in a chamber 60 feet below the 1800-year-old pyramid known as the "Temple of the Feathered Serpent
The Temple of the Feathered Serpent is the third largest pyramid at Teotihuacan, a Pre-Columbian Mexico, pre-Columbian site in central Mexico (the term ''Teotihuacan'', or ''Teotihuacano'', is also used for the whole civilization and cultural com ...
," "the third largest pyramid of Teotihuacan
Teotihuacan (Spanish language, Spanish: ''Teotihuacán'') (; ) is an ancient Mesoamerican city located in a sub-valley of the Valley of Mexico, which is located in the State of Mexico, northeast of modern-day Mexico City. Teotihuacan is ...
," Mexico along with "jade statues, jaguar remains, a box filled with carved shells and rubber balls".
Aristotle
Aristotle (; grc-gre, Ἀριστοτέλης ''Aristotélēs'', ; 384–322 BC) was a Greek philosopher and polymath during the Classical period in Ancient Greece. Taught by Plato, he was the founder of the Peripatetic school of phil ...
recounts that Daedalus
In Greek mythology, Daedalus (, ; Greek: Δαίδαλος; Latin: ''Daedalus''; Etruscan: ''Taitale'') was a skillful architect and craftsman, seen as a symbol of wisdom, knowledge and power. He is the father of Icarus, the uncle of Perdix, an ...
made a wooden statue of Venus
Venus is the second planet from the Sun. It is sometimes called Earth's "sister" or "twin" planet as it is almost as large and has a similar composition. As an interior planet to Earth, Venus (like Mercury) appears in Earth's sky never fa ...
move by pouring quicksilver in its interior. In Greek mythology
A major branch of classical mythology, Greek mythology is the body of myths originally told by the Ancient Greece, ancient Greeks, and a genre of Ancient Greek folklore. These stories concern the Cosmogony, origin and Cosmology#Metaphysical co ...
Daedalus gave the appearance of voice in his statues using quicksilver. The ancient Greeks
Ancient Greece ( el, Ἑλλάς, Hellás) was a northeastern Mediterranean civilization, existing from the Greek Dark Ages of the 12th–9th centuries BC to the end of classical antiquity ( AD 600), that comprised a loose collection of cultu ...
used cinnabar
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of Mercury sulfide, mercury(II) sulfide (HgS). It is the most common source ore for refining mercury (element), elemental mercury and ...
(mercury sulfide) in ointments; the ancient Egyptians and the Romans
Roman or Romans most often refers to:
*Rome, the capital city of Italy
* Ancient Rome, Roman civilization from 8th century BC to 5th century AD
*Roman people, the people of ancient Rome
*''Epistle to the Romans'', shortened to ''Romans'', a lette ...
used it in cosmetics
Cosmetics are constituted mixtures of chemical compounds derived from either natural sources, or synthetically created ones. Cosmetics have various purposes. Those designed for personal care and skin care can be used to cleanse or protect ...
. In Lamanai
Lamanai (from ''Lama'anayin'', "submerged crocodile" in Yucatec Maya) is a Mesoamerican archaeological site, and was once a major city of the Maya civilization, located in the north of Belize, in Orange Walk District. The site's name is pre-Columb ...
, once a major city of the Maya civilization
The Maya civilization () of the Mesoamerican people is known by its ancient temples and glyphs. Its Maya script is the most sophisticated and highly developed writing system in the pre-Columbian Americas. It is also noted for its art, archit ...
, a pool of mercury was found under a marker in a Mesoamerican ballcourt
A Mesoamerican ballcourt ( nah, tlachtli) is a large masonry structure of a type used in Mesoamerica for over 2,700 years to play the Mesoamerican ballgame, particularly the hip-ball version of the ballgame. More than 1,300 ballcourts have been i ...
. By 500 BC mercury was used to make amalgams
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital, an in ...
(Medieval Latin ''amalgama'', "alloy of mercury") with other metals.
Alchemists
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
thought of mercury as the First Matter from which all metals were formed. They believed that different metals
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
could be produced by varying the quality and quantity of sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
contained within the mercury. The purest of these was gold, and mercury was called for in attempts at the transmutation of base (or impure) metals into gold, which was the goal of many alchemists.
The mines in Almadén
Almadén () is a town and municipality in the Spanish province of Ciudad Real, within the autonomous community of Castile-La Mancha. The town is located at 4° 49' W and 38° 46' N and is 589 meters above sea level. Almadén is approximately 300 ...
(Spain), Monte Amiata
Mount Amiata is the largest of the lava domes in the Amiata lava dome complex located about 20 km northwest of Lake Bolsena in the southern Tuscany region of Italy. It is located within the provinces of Grosseto and Siena.
Geology
Mount Am ...
(Italy), and Idrija
Idrija (, in older sources ''Zgornja Idrija''; german: (Ober)idria, it, Idria) is a town in western Slovenia. It is the seat of the Municipality of Idrija. It is located in the traditional region of Inner Carniola and is in the Gorizia Statisti ...
(now Slovenia) dominated mercury production from the opening of the mine in Almadén 2500 years ago, until new deposits were found at the end of the 19th century.
Occurrence
Mercury is an extremely rare element in Earth's crust, having an average crustal abundance by mass of only 0.08 parts per million (ppm). Because it does not blend geochemically with those elements that constitute the majority of the crustal mass, mercury ores can be extraordinarily concentrated considering the element's abundance in ordinary rock. The richest mercury ores contain up to 2.5% mercury by mass, and even the leanest concentrated deposits are at least 0.1% mercury (12,000 times average crustal abundance). It is found either as anative metal
A native metal is any metal that is found pure in its metallic form in nature. Metals that can be found as native deposits singly or in alloys include aluminium, antimony, arsenic, bismuth, cadmium, chromium, cobalt, indium, iron, manganese, m ...
(rare) or in cinnabar
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of Mercury sulfide, mercury(II) sulfide (HgS). It is the most common source ore for refining mercury (element), elemental mercury and ...
, metacinnabar, sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimen ...
, corderoite
Corderoite is an extremely rare mercury sulfide chloride mineral with formula Hg3S2Cl2. It crystallizes in the isometric crystal system. It is soft, 1.5 to 2 on the Mohs scale, and varies in color from light gray to black and rarely pink or yell ...
, livingstonite and other mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. ( ...
s, with cinnabar (HgS) being the most common ore. Mercury ores often occur in hot springs or other volcanic
A volcano is a rupture in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often found where tectonic plates a ...
regions.
Beginning in 1558, with the invention of the patio process
The patio process is a process for extracting silver from ore. Smelting, or refining, was necessary because silver does not occur by itself in a natural state like some metals. Instead, it is made up of a larger ore body. Thus, smelting, or refin ...
to extract silver from ore using mercury, mercury became an essential resource in the economy of Spain and its American colonies. Mercury was used to extract silver from the lucrative mines in New Spain
New Spain, officially the Viceroyalty of New Spain ( es, Virreinato de Nueva España, ), or Kingdom of New Spain, was an integral territorial entity of the Spanish Empire, established by Habsburg Spain during the Spanish colonization of the Am ...
and Peru
, image_flag = Flag of Peru.svg
, image_coat = Escudo nacional del Perú.svg
, other_symbol = Great Seal of the State
, other_symbol_type = Seal (emblem), National seal
, national_motto = "Fi ...
. Initially, the Spanish Crown's mines in Almadén in Southern Spain supplied all the mercury for the colonies. Mercury deposits were discovered in the New World, and more than 100,000 tons of mercury were mined from the region of Huancavelica
Huancavelica () or Wankawillka in Quechua is a city in Peru. It is the capital of the department of Huancavelica and according to the 2017 census had a population of 49,570 people. The city was established on August 5, 1572 by the Viceroy ...
, Peru, over the course of three centuries following the discovery of deposits there in 1563. The patio process and later pan amalgamation The pan amalgamation process is a method to extract silver from ore, using salt and copper(II) sulfate in addition to mercury. The process was widely used from 1609 through the 19th century; it is no longer used.
The patio process had been used to ...
process continued to create great demand for mercury to treat silver ores until the late 19th century.
 Former mines in Italy, the United States and Mexico, which once produced a large proportion of the world supply, have now been completely mined out or, in the case of Slovenia (
Former mines in Italy, the United States and Mexico, which once produced a large proportion of the world supply, have now been completely mined out or, in the case of Slovenia (Idrija
Idrija (, in older sources ''Zgornja Idrija''; german: (Ober)idria, it, Idria) is a town in western Slovenia. It is the seat of the Municipality of Idrija. It is located in the traditional region of Inner Carniola and is in the Gorizia Statisti ...
) and Spain (Almadén
Almadén () is a town and municipality in the Spanish province of Ciudad Real, within the autonomous community of Castile-La Mancha. The town is located at 4° 49' W and 38° 46' N and is 589 meters above sea level. Almadén is approximately 300 ...
), shut down due to the fall of the price of mercury. Nevada
Nevada ( ; ) is a U.S. state, state in the Western United States, Western region of the United States. It is bordered by Oregon to the northwest, Idaho to the northeast, California to the west, Arizona to the southeast, and Utah to the east. N ...
's McDermitt Mine, the last mercury mine in the United States, closed in 1992. The price of mercury has been highly volatile over the years and in 2006 was $650 per 76-pound (34.46 kg) flask
Flask may refer to:
Container
* Hip flask, a small container used to carry a small amount of liquid
* Laboratory flask, laboratory glassware for holding larger volumes than simple test tubes
** Erlenmeyer flask, a common laboratory flask wit ...
.
Mercury is extracted by heating cinnabar in a current of air and condensing the vapor. The equation for this extraction is
:HgS + O2 → Hg + SO2
In 2005, China was the top producer of mercury with almost two-thirds global share followed by Kyrgyzstan
Kyrgyzstan,, pronounced or the Kyrgyz Republic, is a landlocked country in Central Asia. Kyrgyzstan is bordered by Kazakhstan to the north, Uzbekistan to the west, Tajikistan to the south, and the People's Republic of China to the east. ...
. Several other countries are believed to have unrecorded production of mercury from copper electrowinning
Electrowinning, also called electroextraction, is the electrodeposition of metals from their ores that have been put in solution via a process commonly referred to as leaching. Electrorefining uses a similar process to remove impurities from a ...
processes and by recovery from effluents.
Because of the high toxicity of mercury, both the mining of cinnabar and refining for mercury are hazardous and historic causes of mercury poisoning. In China, prison labor was used by a private mining company as recently as the 1950s to develop new cinnabar mines. Thousands of prisoners were used by the Luo Xi mining company to establish new tunnels. Worker health in functioning mines is at high risk.
A newspaper claimed that an unidentified European Union
The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been des ...
directive calling for energy-efficient lightbulbs to be made mandatory by 2012 encouraged China to re-open cinnabar mines to obtain the mercury required for CFL bulb manufacture. Environmental dangers have been a concern, particularly in the southern cities of Foshan
Foshan (, ), alternately romanized as Fatshan, is a prefecture-level city in central Guangdong Province, China. The entire prefecture covers and had a population of 9,498,863 as of the 2020 census. The city is part of the western side of the ...
and Guangzhou
Guangzhou (, ; ; or ; ), also known as Canton () and alternatively romanized as Kwongchow or Kwangchow, is the capital and largest city of Guangdong province in southern China. Located on the Pearl River about north-northwest of Hong Kon ...
, and in Guizhou
Guizhou (; formerly Kweichow) is a landlocked province in the southwest region of the People's Republic of China. Its capital and largest city is Guiyang, in the center of the province. Guizhou borders the autonomous region of Guangxi to t ...
province in the southwest.
Abandoned mercury mine processing sites often contain very hazardous waste piles of roasted cinnabar calcine
Calcination refers to thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), gener ...
s. Water run-off from such sites is a recognized source of ecological damage. Former mercury mines may be suited for constructive re-use. For example, in 1976 Santa Clara County, California
Santa Clara County, officially the County of Santa Clara, is the sixth-most populous county in the U.S. state of California, with a population of 1,936,259, as of the 2020 United States Census, 2020 census. Santa Clara County and neighboring Sa ...
purchased the historic Almaden Quicksilver Mine and created a county park on the site, after conducting extensive safety and environmental analysis of the property.
Chemistry
All known mercury compounds exhibit one of two positive oxidation states: I and II. Experiments have failed to unequivocally demonstrate any higher oxidation states: both the claimed 1976 electrosynthesis of an unstable Hg(III) species and 2007 cryogenic isolation of HgF4 have disputed interpretations and remain difficult (if not impossible) to reproduce.Compounds of mercury(I)
Unlike its lighter neighbors, cadmium and zinc, mercury usually forms simple stable compounds with metal-metal bonds. Most mercury(I) compounds arediamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
and feature the dimeric cation, Hg. Stable derivatives include the chloride and nitrate. Treatment of Hg(I) compounds complexation with strong ligands such as sulfide, cyanide, etc. induces disproportionation to and elemental mercury. Mercury(I) chloride
Mercury(I) chloride is the chemical compound with the formula Hg2Cl2. Also known as the mineral calomel (a rare mineral) or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury(I) compound. ...
, a colorless solid also known as calomel
Calomel is a mercury chloride mineral with formula Hg2Cl2 (see mercury(I) chloride). The name derives from Greek ''kalos'' (beautiful) and ''melas'' (black) because it turns black on reaction with ammonia. This was known to alchemists.
Calomel ...
, is really the compound with the formula Hg2Cl2, with the connectivity Cl-Hg-Hg-Cl. It is a standard in electrochemistry. It reacts with chlorine to give mercuric chloride, which resists further oxidation. Mercury(I) hydride, a colorless gas, has the formula HgH, containing no Hg-Hg bond.
Indicative of its tendency to bond to itself, mercury forms mercury polycations, which consist of linear chains of mercury centers, capped with a positive charge. One example is .
Compounds of mercury(II)
Mercury(II) is the most common oxidation state and is the main one in nature as well. All four mercuric halides are known. They form tetrahedral complexes with other ligands but the halides adopt linear coordination geometry, somewhat like Ag+ does. Best known ismercury(II) chloride
Mercury(II) chloride (or mercury bichloride, mercury dichloride), historically also known as sulema or corrosive sublimate, is the inorganic chemical compound of mercury and chlorine with the formula HgCl2. It is white crystalline solid and is ...
, an easily sublimating white solid. HgCl2 forms coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es that are typically tetrahedral, e.g. .
Mercury(II) oxide
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is v ...
, the main oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
of mercury, arises when the metal is exposed to air for long periods at elevated temperatures. It reverts to the elements upon heating near 400 °C, as was demonstrated by Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted exp ...
in an early synthesis of pure oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
. Hydroxides of mercury are poorly characterized, as they are for its neighbors gold and silver.
Being a soft metal, mercury forms very stable derivatives with the heavier chalcogens
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioa ...
. Preeminent is mercury(II) sulfide
Mercury sulfide, or mercury(II) sulfide is a chemical compound composed of the chemical elements mercury (element), mercury and sulfur. It is represented by the chemical formula HgS. It is virtually insoluble in water.
Crystal structure
HgS ...
, HgS, which occurs in nature as the ore cinnabar
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of Mercury sulfide, mercury(II) sulfide (HgS). It is the most common source ore for refining mercury (element), elemental mercury and ...
and is the brilliant pigment vermillion
Vermilion (sometimes vermillion) is a color, color family, and pigment most often made, since antiquity until the 19th century, from the powdered mineral cinnabar (a form of mercury sulfide, which is toxic) and its corresponding color. It is v ...
. Like ZnS, HgS crystallizes in two forms
Form is the shape, visual appearance, or configuration of an object. In a wider sense, the form is the way something happens.
Form also refers to:
*Form (document), a document (printed or electronic) with spaces in which to write or enter data
* ...
, the reddish cubic form and the black zinc blende
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Va ...
form. The latter sometimes occurs naturally as metacinnabar. Mercury(II) selenide (HgSe) and mercury(II) telluride
Mercury telluride (HgTe) is a binary chemical compound of mercury and tellurium. It is a semi-metal related to the II-VI group of semiconductor materials. Alternative names are mercuric telluride and mercury(II) telluride.
HgTe occurs in nature ...
(HgTe) are also known, these as well as various derivatives, e.g. mercury cadmium telluride Hg1−xCdxTe or mercury cadmium telluride (also cadmium mercury telluride, MCT, MerCad Telluride, MerCadTel, MerCaT or CMT) is a chemical compound of cadmium telluride (CdTe) and mercury telluride (HgTe) with a tunable bandgap spanning the shortwav ...
and mercury zinc telluride Mercury zinc telluride (HgZnTe, MZT) is a telluride of mercury and zinc, an alloy of mercury telluride and zinc telluride. It is a narrow-gap semiconductor material.
Mercury zinc telluride is used in infrared detectors and arrays for infrared i ...
being semiconductors
A semiconductor is a material which has an electrical resistivity and conductivity, electrical conductivity value falling between that of a electrical conductor, conductor, such as copper, and an insulator (electricity), insulator, such as glas ...
useful as infrared detector
An infrared detector is a detector that reacts to infrared (IR) radiation. The two main types of detectors are thermal and photonic (photodetectors).
The thermal effects of the incident IR radiation can be followed through many temperature depen ...
materials.
Mercury(II) salts form a variety of complex derivatives with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
. These include Millon's base (Hg2N+), the one-dimensional polymer (salts of )), and "fusible white precipitate" or g(NH3)2l2. Known as Nessler's reagent, potassium tetraiodomercurate(II)
Potassium tetraiodomercurate(II) is an inorganic compound consisting of potassium cations and the tetraiodomercurate(II) anion. It is mainly used as Nessler's reagent, a 0.09 mol/L solution of potassium tetraiodomercurate(II) (K2 gI4 in 2.5&n ...
() is still occasionally used to test for ammonia owing to its tendency to form the deeply colored iodide salt of Millon's base.
Mercury fulminate
Mercury(II) fulminate, or Hg(CNO)2, is a primary explosive. It is highly sensitive to friction, heat and shock and is mainly used as a trigger for other explosives in percussion caps and detonators. Mercury(II) cyanate, though its chemical formu ...
is a detonator widely used in explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An expl ...
s.
Organomercury compounds
Organic mercury compounds are historically important but are of little industrial value in the western world. Mercury(II) salts are a rare example of simple metal complexes that react directly with aromatic rings. Organomercury compounds are always divalent and usually two-coordinate and linear geometry. Unlikeorganocadmium
An organocadmium compound is an organometallic compound containing a carbon to cadmium chemical bond. Organocadmium chemistry describes physical properties, synthesis, reactions and use of these compounds. Cadmium shares group 12 with zinc and me ...
and organozinc
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compoun ...
compounds, organomercury compounds do not react with water. They usually have the formula HgR2, which are often volatile, or HgRX, which are often solids, where R is aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
or alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
and X is usually halide or acetate. Methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a ...
, a generic term for compounds with the formula CH3HgX, is a dangerous family of compounds that are often found in polluted
Pollution is the introduction of contaminants into the natural environment that cause adverse change. Pollution can take the form of any substance (solid, liquid, or gas) or energy (such as radioactivity, heat, sound, or light). Pollutants, the ...
water. They arise by a process known as biomethylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These ...
.
Applications
fluorescent lamps
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet ligh ...
, while most of the other applications are slowly being phased out due to health and safety regulations. In some applications, mercury is replaced with less toxic but considerably more expensive Galinstan
Galinstan (R) is a brand name for a alloy composed of gallium, indium, and tin which melts at and is thus liquid at room temperature. However, it is not a eutectic alloy but a near eutectic alloy. In scientific literature, galinstan is also used ...
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, ...
.
Medicine
 Mercury and its compounds have been used in medicine, although they are much less common today than they once were, now that the toxic effects of mercury and its compounds are more widely understood. An example of the early therapeutic application of mercury of was published in 1787 by
Mercury and its compounds have been used in medicine, although they are much less common today than they once were, now that the toxic effects of mercury and its compounds are more widely understood. An example of the early therapeutic application of mercury of was published in 1787 by James Lind
James Lind (4 October 1716 – 13 July 1794) was a Scottish doctor. He was a pioneer of naval hygiene in the Royal Navy. By conducting one of the first ever clinical trials, he developed the theory that citrus fruits cured scurvy.
Lind arg ...
.
The first edition of the Merck's Manual (1899) featured many mercuric compounds such as:
* Mercauro
* Mercuro-iodo-hemol.
* Mercury-ammonium chloride
* Mercury Benzoate
* Mercuric
* Mercury Bichloride (Corrosive Mercuric Chloride, U.S.P.)
* Mercury Chloride
* Mild Mercury Cyanide
* Mercury Succinimide
* Mercury Iodide
* Red Mercury Biniodide
* Mercury Iodide
* Yellow Mercury Proto-iodide
* Black (Hahnemann), Soluble Mercury Oxide
* Red Mercury Oxide
* Yellow Mercury Oxide
* Mercury Salicylate
* Mercury Succinimide
* Mercury Imido-succinate
* Mercury Sulphate
* Basic Mercury Subsulphate; Turpeth Mineral
* Mercury Tannate
* Mercury-Ammonium Chloride
Mercury is an ingredient in dental amalgams. Thiomersal
Thiomersal (INN), or thimerosal (USAN, JAN), is an organomercury compound. It is a well-established antiseptic and antifungal agent.
The pharmaceutical corporation Eli Lilly and Company gave thiomersal the trade name Merthiolate. It has been u ...
(called ''Thimerosal'' in the United States) is an organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
used as a preservative
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or by ...
in vaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifie ...
s, though this use is in decline. Thiomersal is metabolized to ethyl mercury. Although it was widely speculated that this mercury-based preservative could cause or trigger autism
The autism spectrum, often referred to as just autism or in the context of a professional diagnosis autism spectrum disorder (ASD) or autism spectrum condition (ASC), is a neurodevelopmental condition (or conditions) characterized by difficulti ...
in children, scientific studies showed no evidence supporting any such link. Nevertheless, thiomersal has been removed from, or reduced to trace amounts in all U.S. vaccines recommended for children 6 years of age and under, with the exception of inactivated influenza vaccine.
Another mercury compound, merbromin
Merbromin (marketed as Mercurochrome, Merbromine, Mercurocol, Sodium mercurescein, Asceptichrome, Supercrome, Brocasept and Cinfacromin) is an organomercuric disodium salt compound used as a topical antiseptic for minor cuts and scrapes and a ...
(Mercurochrome), is a topical antiseptic used for minor cuts and scrapes that is still in use in some countries.
Mercury in the form of one of its common ores, cinnabar, is used in various traditional medicines, especially in traditional Chinese medicine
Traditional Chinese medicine (TCM) is an alternative medical practice drawn from traditional medicine in China. It has been described as "fraught with pseudoscience", with the majority of its treatments having no logical mechanism of action ...
. Review of its safety has found that cinnabar can lead to significant mercury intoxication when heated, consumed in overdose
A drug overdose (overdose or OD) is the ingestion or application of a drug or other substance in quantities much greater than are recommended.
, or taken long term, and can have adverse effects at therapeutic doses, though effects from therapeutic doses are typically reversible. Although this form of mercury appears to be less toxic than other forms, its use in traditional Chinese medicine has not yet been justified, as the therapeutic basis for the use of cinnabar is not clear.
Today, the use of mercury in medicine has greatly declined in all respects, especially in developed countries. Thermometers
A thermometer is a device that measures temperature or a temperature gradient (the degree of hotness or coldness of an object). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb of a mercury-in-glass thermomete ...
and sphygmomanometer
A sphygmomanometer ( ), a blood pressure monitor, or blood pressure gauge, is a device used to measure blood pressure, composed of an inflatable cuff to collapse and then release the artery under the cuff in a controlled manner, and a mercury (e ...
s containing mercury were invented in the early 18th and late 19th centuries, respectively. In the early 21st century, their use is declining and has been banned in some countries, states and medical institutions. In 2002, the U.S. Senate
The United States Senate is the upper chamber of the United States Congress, with the House of Representatives being the lower chamber. Together they compose the national bicameral legislature of the United States.
The composition and powe ...
passed legislation to phase out the sale of non-prescription mercury thermometers. In 2003, Washington
Washington commonly refers to:
* Washington (state), United States
* Washington, D.C., the capital of the United States
** A metonym for the federal government of the United States
** Washington metropolitan area, the metropolitan area centered o ...
and Maine
Maine () is a state in the New England and Northeastern regions of the United States. It borders New Hampshire to the west, the Gulf of Maine to the southeast, and the Canadian provinces of New Brunswick and Quebec to the northeast and north ...
became the first states to ban mercury blood pressure devices. Mercury compounds are found in some over-the-counter drug
Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs
A prescription drug (also prescription medication or prescripti ...
s, including topical antiseptics
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putre ...
, stimulant laxatives, diaper-rash ointment, eye drops
Eye drops or eyedrops are liquid drops applied directly to the surface of the eye usually in small amounts such as a single drop or a few drops. Eye drops usually contain saline to match the salinity of the eye. Drops containing only saline ...
, and nasal spray
Nasal sprays are used to deliver medications locally in the nasal cavities or systemically. They are used locally for conditions such as nasal congestion and allergic rhinitis. In some situations, the nasal delivery route is preferred for syste ...
s. The FDA
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
has "inadequate data to establish general recognition of the safety and effectiveness" of the mercury ingredients in these products. Mercury is still used in some diuretics although substitutes now exist for most therapeutic uses.
Production of chlorine and caustic soda
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
is produced from sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
(common salt, NaCl) using electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
to separate the metallic sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
from the chlorine gas. Usually the salt is dissolved in water to produce a brine. By-products of any such chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are comm ...
are hydrogen (H2) and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
(NaOH), which is commonly called caustic soda or lye
A lye is a metal hydroxide traditionally obtained by leaching wood ashes, or a strong alkali which is highly soluble in water producing caustic basic solutions. "Lye" most commonly refers to sodium hydroxide (NaOH), but historically has been u ...
. By far the largest use of mercury in the late 20th century was in the mercury cell process (also called the Castner-Kellner process) where metallic sodium is formed as an amalgam
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital
...
at a cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
made from mercury; this sodium is then reacted with water to produce sodium hydroxide. Many of the industrial mercury releases of the 20th century came from this process, although modern plants claimed to be safe in this regard. After about 1985, all new chloralkali production facilities that were built in the United States used membrane cell or diaphragm cell technologies to produce chlorine.
Laboratory uses
Some medical thermometers, especially those for high temperatures, are filled with mercury; they are gradually disappearing. In the United States, non-prescription sale of mercury fever thermometers has been banned since 2003. Some transit telescopes use a basin of mercury to form a flat and absolutely horizontal mirror, useful in determining an absolute vertical or perpendicular reference. Concave horizontal parabolic mirrors may be formed by rotating liquid mercury on a disk, the parabolic form of the liquid thus formed reflecting and focusing incident light. Suchliquid-mirror telescope
Liquid-mirror telescopes are telescopes with mirrors made with a reflective liquid. The most common liquid used is mercury, but other liquids will work as well (for example, low-melting alloys of gallium). The liquid and its container are rotated ...
s are cheaper than conventional large mirror telescopes by up to a factor of 100, but the mirror cannot be tilted and always points straight up.
Liquid mercury is a part of popular secondary reference electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials de ...
(called the calomel electrode
The saturated calomel electrode (SCE) is a reference electrode based on the reaction between elemental Mercury (element), mercury and mercury(I) chloride. It has been widely replaced by the silver chloride electrode, however the calomel electrode ...
) in electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
as an alternative to the standard hydrogen electrode
The standard hydrogen electrode (abbreviated SHE), is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials. Its absolute electrode potential is estimated to be at 25 °C, but to form a basis f ...
. The calomel electrode is used to work out the electrode potential
In electrochemistry, electrode potential is the electromotive force of a galvanic cell built from a standard reference electrode and another electrode to be characterized. By convention, the reference electrode is the standard hydrogen electrode (S ...
of half cell
In electrochemistry, a half-cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electri ...
s. Last, but not least, the triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the subli ...
of mercury, −38.8344 °C, is a fixed point used as a temperature standard for the International Temperature Scale (ITS-90
The International Temperature Scale of 1990 (ITS-90) is an equipment calibration standard specified by the International Committee of Weights and Measures (CIPM) for making measurements on the Kelvin and Celsius temperature scales. It is an approx ...
).
In polarography
Polarography is a type of voltammetry where the working electrode is a dropping mercury electrode (DME) or a static mercury drop electrode (SMDE), which are useful for their wide cathodic ranges and renewable surfaces. It was invented in 1922 by ...
both the dropping mercury electrode
A liquid metal electrode is an electrode that uses a liquid metal, such as mercury, Galinstan, and NaK. They can be used in electrocapillarity, voltammetry, and impedance measurements.
Dropping mercury electrode
The dropping mercury electrode ...
and the hanging mercury drop electrode
A liquid metal electrode is an electrode that uses a liquid metal, such as mercury, Galinstan, and NaK. They can be used in electrocapillarity, voltammetry, and impedance measurements.
Dropping mercury electrode
The dropping mercury electrode ...
use elemental mercury. This use allows a new uncontaminated electrode to be available for each measurement or each new experiment.
Mercury-containing compounds are also of use in the field of structural biology
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every le ...
. Mercuric compounds such as mercury(II) chloride
Mercury(II) chloride (or mercury bichloride, mercury dichloride), historically also known as sulema or corrosive sublimate, is the inorganic chemical compound of mercury and chlorine with the formula HgCl2. It is white crystalline solid and is ...
or potassium tetraiodomercurate(II)
Potassium tetraiodomercurate(II) is an inorganic compound consisting of potassium cations and the tetraiodomercurate(II) anion. It is mainly used as Nessler's reagent, a 0.09 mol/L solution of potassium tetraiodomercurate(II) (K2 gI4 in 2.5&n ...
can be added to protein crystals in an effort to create heavy atom derivatives that can be used to solve the phase problem
In physics, the phase problem is the problem of loss of information concerning the phase that can occur when making a physical measurement. The name comes from the field of X-ray crystallography, where the phase problem has to be solved for the det ...
in X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
via isomorphous replacement Multiple isomorphous replacement (MIR) is historically the most common approach to solving the phase problem in X-ray crystallography studies of proteins. For protein crystals this method is conducted by soaking the crystal of a sample to be analyze ...
or anomalous scattering Anomalous X-ray scattering (AXRS or XRAS) is a non-destructive determination technique within X-ray diffraction that makes use of the anomalous dispersion that occurs when a wavelength is selected that is in the vicinity of an absorption edge of on ...
methods.
Niche uses
Gaseous mercury is used inmercury-vapor lamp
A mercury-vapor lamp is a gas-discharge lamp that uses an electric arc through vaporized mercury to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger soda lime or borosilicate glass ...
s and some "neon sign
In the signage industry, neon signs are electric signs lighted by long luminous gas-discharge tubes that contain rarefied neon or other gases. They are the most common use for neon lighting, which was first demonstrated in a modern form in Decem ...
" type advertising signs and fluorescent lamp
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet lig ...
s. Those low-pressure lamps emit very spectrally narrow lines, which are traditionally used in optical spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wav ...
for calibration of spectral position. Commercial calibration lamps are sold for this purpose; reflecting a fluorescent ceiling light into a spectrometer is a common calibration practice. Gaseous mercury is also found in some electron tubes, including ignitron
An ignitron is a type of gas-filled tube used as a controlled rectifier and dating from the 1930s. Invented by Joseph Slepian while employed by Westinghouse, Westinghouse was the original manufacturer and owned trademark rights to the name "Ignit ...
s, thyratron
A thyratron is a type of gas-filled tube used as a high-power electrical switch and controlled rectifier. Thyratrons can handle much greater currents than similar hard-vacuum tubes. Electron multiplication occurs when the gas becomes ionized, pro ...
s, and mercury arc rectifier
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Mercury ...
s. It is also used in specialist medical care lamps for skin tanning and disinfection. Gaseous mercury is added to cold cathode
A cold cathode is a cathode that is not electrically heated by a filament.A negatively charged electrode emits electrons or is the positively charged terminal. For more, see field emission. A cathode may be considered "cold" if it emits more el ...
argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
-filled lamps to increase the ionization
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
and electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
. An argon-filled lamp without mercury will have dull spots and will fail to light correctly. Lighting containing mercury can be bombarded/oven pumped only once. When added to neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton ...
filled tubes the light produced will be inconsistent red/blue spots until the initial burning-in process is completed; eventually it will light a consistent dull off-blue color.
germicidal lamp
A germicidal lamp (also known as disinfection lamp or sterilizer lamp) is an electric light that produces ultraviolet C (UVC) light. This short-wave ultraviolet light disrupts DNA base pairing, causing formation of pyrimidine dimers, and lead ...
, whose spectrum is rich in invisible ultraviolet radiation.
File:Mercuryvaporlamp.jpg, Skin tanner containing a low-pressure mercury vapor lamp and two infrared lamps, which act both as light source and electrical ballast
An electrical ballast is a device placed in series with a load to limit the amount of electric current, current in an electrical network, electrical circuit.
A familiar and widely used example is the inductive ballast used in fluorescent lamp ...
File:Leuchtstofflampen-chtaube050409.jpg, Assorted types of fluorescent lamps.
File:Deep Space Atomic Clock-DSAC.jpg, The miniaturized Deep Space Atomic Clock is a linear ion-trap-based mercury ion clock, designed for precise and real-time radio navigation in deep space.
Jet Propulsion Laboratory
The Jet Propulsion Laboratory (JPL) is a federally funded research and development center and NASA field center in the City of La Cañada Flintridge, California, United States.
Founded in the 1930s by Caltech researchers, JPL is owned by NASA an ...
utilises mercury in a linear ion-trap-based clock. The novel use of mercury allows very compact atomic clocks, with low energy requirements, and is therefore ideal for space probes and Mars missions.
Cosmetics
Mercury, asthiomersal
Thiomersal (INN), or thimerosal (USAN, JAN), is an organomercury compound. It is a well-established antiseptic and antifungal agent.
The pharmaceutical corporation Eli Lilly and Company gave thiomersal the trade name Merthiolate. It has been u ...
, is widely used in the manufacture of mascara
Mascara is a cosmetic commonly used to enhance the upper and lower eyelashes. It is used to darken, thicken, lengthen, and/or define the eyelashes. Normally in one of three forms—liquid, powder, or cream—the modern mascara product has vario ...
. In 2008, Minnesota became the first state in the United States to ban intentionally added mercury in cosmetics, giving it a tougher standard than the federal government.
A study in geometric mean urine mercury concentration identified a previously unrecognized source of exposure (skin care products) to inorganic mercury among New York City
New York, often called New York City or NYC, is the List of United States cities by population, most populous city in the United States. With a 2020 population of 8,804,190 distributed over , New York City is also the L ...
residents. Population-based biomonitoring also showed that mercury concentration levels are higher in consumers of seafood and fish meals.
Skin whitening
Mercury is effective as an active ingredient inskin whitening
Skin whitening, also known as skin lightening and skin bleaching, is the practice of using chemical substances in an attempt to lighten the skin or provide an even skin color by reducing the melanin concentration in the skin. Several chemicals ha ...
compounds used to depigment skin. The Minamata Convention on Mercury
The Minamata Convention on Mercury is an international treaty designed to protect human health and the environment from anthropogenic emissions and releases of mercury and mercury compounds. The convention was a result of three years of meetin ...
limits the concentration of mercury in such whiteners to 1 part per million. However, as of 2022, many commercially sold whitener products continue to exceed that limit, and are considered toxic.
Firearms
Mercury(II) fulminate
Mercury(II) fulminate, or Hg(CNO)2, is a primary explosive. It is highly sensitive to friction, heat and shock and is mainly used as a trigger for other explosives in percussion caps and detonators. Mercury(II) cyanate, though its chemical formula ...
is a primary explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An expl ...
which is mainly used as a primer
Primer may refer to:
Arts, entertainment, and media Films
* ''Primer'' (film), a 2004 feature film written and directed by Shane Carruth
* ''Primer'' (video), a documentary about the funk band Living Colour
Literature
* Primer (textbook), a t ...
of a cartridge
Cartridge may refer to:
Objects
* Cartridge (firearms), a type of modern ammunition
* ROM cartridge, a removable component in an electronic device
* Cartridge (respirator), a type of filter used in respirators
Other uses
* Cartridge (surname), a ...
in firearms.
Historic uses

 Many historic applications made use of the peculiar physical properties of mercury, especially as a dense liquid and a liquid metal:
* Quantities of liquid mercury ranging from have been recovered from elite
Many historic applications made use of the peculiar physical properties of mercury, especially as a dense liquid and a liquid metal:
* Quantities of liquid mercury ranging from have been recovered from elite Maya
Maya may refer to:
Civilizations
* Maya peoples, of southern Mexico and northern Central America
** Maya civilization, the historical civilization of the Maya peoples
** Maya language, the languages of the Maya peoples
* Maya (Ethiopia), a populat ...
tombs (100–700 AD) or ritual caches at six sites. This mercury may have been used in bowls as mirrors
A mirror or looking glass is an object that Reflection (physics), reflects an image. Light that bounces off a mirror will show an image of whatever is in front of it, when focused through the lens of the eye or a camera. Mirrors reverse the ...
for divinatory
Divination (from Latin ''divinare'', 'to foresee, to foretell, to predict, to prophesy') is the attempt to gain insight into a question or situation by way of an occultic, standardized process or ritual. Used in various forms throughout histor ...
purposes. Five of these date to the Classic Period of Maya civilization (c. 250–900) but one example predated this.
* In Islamic Spain
Al-Andalus translit. ; an, al-Andalus; ast, al-Ándalus; eu, al-Andalus; ber, ⴰⵏⴷⴰⵍⵓⵙ, label= Berber, translit=Andalus; ca, al-Àndalus; gl, al-Andalus; oc, Al Andalús; pt, al-Ândalus; es, al-Ándalus () was the Mu ...
, it was used for filling decorative pools. Later, the American artist Alexander Calder
Alexander Calder (; July 22, 1898 – November 11, 1976) was an American sculptor known both for his innovative mobiles (kinetic sculptures powered by motors or air currents) that embrace chance in their aesthetic, his static "stabiles", and his ...
built a mercury fountain
A mercury fountain is a fountain constructed for use with liquid metallic mercury ("quicksilver") rather than water.
Mercury fountains existed in some castles in Islamic Spain; the most famous one was located at the Kasr-al-Kholaifa in Córdob ...
for the Spanish Pavilion at the 1937 World Exhibition in Paris. The fountain is now on display at the Fundació Joan Miró
The Fundació Joan Miró ( ; "Joan Miró Foundation, Centre of Studies of Contemporary Art") is a museum of modern art honoring Joan Miró located on the hill called Montjuïc in Barcelona, Catalonia (Spain).
History
The idea for the foundation ...
in Barcelona
Barcelona ( , , ) is a city on the coast of northeastern Spain. It is the capital and largest city of the autonomous community of Catalonia, as well as the second most populous municipality of Spain. With a population of 1.6 million within ci ...
.
* Mercury was used inside wobbler lures. Its heavy, liquid form made it useful since the lures made an attractive irregular movement when the mercury moved inside the plug. Such use was stopped due to environmental concerns, but illegal preparation of modern fishing plugs has occurred.
* The Fresnel lens
A Fresnel lens ( ; ; or ) is a type of composite compact lens developed by the French physicist Augustin-Jean Fresnel (1788–1827) for use in lighthouses. It has been called "the invention that saved a million ships."
The design allows the c ...
es of old lighthouses
A lighthouse is a tower, building, or other type of physical structure designed to emit light from a system of lamps and lenses and to serve as a beacon for navigational aid, for maritime pilots at sea or on inland waterways.
Lighthouses mar ...
used to float and rotate in a bath of mercury which acted like a bearing.
* Mercury sphygmomanometer
A sphygmomanometer ( ), a blood pressure monitor, or blood pressure gauge, is a device used to measure blood pressure, composed of an inflatable cuff to collapse and then release the artery under the cuff in a controlled manner, and a mercury (e ...
s (blood pressure meter), barometer
A barometer is a scientific instrument that is used to measure air pressure in a certain environment. Pressure tendency can forecast short term changes in the weather. Many measurements of air pressure are used within surface weather analysis ...
s, diffusion pump
Diffusion pumps use a high speed jet of vapor to direct gas molecules in the pump throat down into the bottom of the pump and out the exhaust. They were the first type of high vacuum pumps operating in the regime of free molecular flow, where the ...
s, coulometers, and many other laboratory instruments took advantage of mercury's properties as a very dense, opaque liquid with a nearly linear thermal expansion.
* As an electrically conductive liquid, it was used in mercury switch
A mercury switch is an electrical switch that opens and closes a circuit when a small amount of the liquid metal mercury connects metal electrodes to close the circuit. There are several different basic designs (tilt, displacement, radial, etc. ...
es (including home mercury light switches installed prior to 1970), tilt switches used in old fire detectors, and tilt switches in some home thermostats.
* Owing to its acoustic properties, mercury was used as the propagation medium in delay-line memory
Delay-line memory is a form of computer memory, now obsolete, that was used on some of the earliest digital computers. Like many modern forms of electronic computer memory, delay-line memory was a refreshable memory, but as opposed to modern ra ...
devices used in early digital computers of the mid-20th century.
* Experimental mercury vapor turbine A mercury vapour turbine is a form of heat engine that uses mercury (element), mercury as the working fluid of its thermal cycle. A mercury vapour turbine has been used in conjunction with a steam turbine for Electrical generator, generating electr ...
s were installed to increase the efficiency of fossil-fuel electrical power plants. The South Meadow power plant in Hartford, CT employed mercury as its working fluid
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, a ...
, in a binary
Binary may refer to:
Science and technology Mathematics
* Binary number, a representation of numbers using only two digits (0 and 1)
* Binary function, a function that takes two arguments
* Binary operation, a mathematical operation that t ...
configuration with a secondary water circuit, for a number of years starting in the late 1920s in a drive to improve plant efficiency. Several other plants were built, including the Schiller Station in Portsmouth, NH, which went online in 1950. The idea did not catch on industry-wide due to the weight and toxicity of mercury, as well as the advent of supercritical steam plants in later years.
* Similarly, liquid mercury was used as a coolant
A coolant is a substance, typically liquid, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosio ...
for some nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
s; however, sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
is proposed for reactors cooled with liquid metal, because the high density of mercury requires much more energy to circulate as coolant.
* Mercury was a propellant for early ion engines
An ion thruster, ion drive, or ion engine is a form of electric propulsion used for spacecraft propulsion. It creates thrust by accelerating ions using electricity.
An ion thruster ionizes a neutral gas by extracting some electrons out of ...
in electric space propulsion systems. Advantages were mercury's high molecular weight, low ionization energy, low dual-ionization energy, high liquid density and liquid storability at room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
. Disadvantages were concerns regarding environmental impact associated with ground testing and concerns about eventual cooling and condensation of some of the propellant on the spacecraft in long-duration operations. The first spaceflight to use electric propulsion was a mercury-fueled ion thruster developed at NASA Glenn Research Center
NASA John H. Glenn Research Center at Lewis Field is a NASA center within the cities of Brook Park and Cleveland between Cleveland Hopkins International Airport and the Rocky River Reservation of Cleveland Metroparks, with a subsidiary facilit ...
and flown on the Space Electric Rocket Test "SERT-1
SERT-1 (Space Electric Rocket Test) was a NASA probe used to test electrostatic ion thruster design and was built by NASA's Lewis Research Center (now NASA Glenn). SERT-1 was the first spacecraft to utilize ion engine design. It was launched on ...
" spacecraft launched by NASA
The National Aeronautics and Space Administration (NASA ) is an independent agency of the US federal government responsible for the civil space program, aeronautics research, and space research.
NASA was established in 1958, succeeding t ...
at its Wallops Flight Facility
Wallops Flight Facility (WFF) is a rocket launch site on Wallops Island on the Eastern Shore of Virginia, United States, just east of the Delmarva Peninsula and approximately north-northeast of Norfolk. The facility is operated by the Goddard ...
in 1964. The SERT-1 flight was followed up by the SERT-2 flight in 1970. Mercury and caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
were preferred propellants for ion engines until Hughes Research Laboratory performed studies finding xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
gas to be a suitable replacement. Xenon is now the preferred propellant for ion engines as it has a high molecular weight, little or no reactivity due to its noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemi ...
nature, and has a high liquid density under mild cryogenic storage.
Other applications made use of the chemical properties of mercury:
* The mercury battery
A mercury battery (also called mercuric oxide battery, mercury cell, button cell, or Ruben-Mallory) is a non-rechargeable electrochemical battery, a primary cell. Mercury batteries use a reaction between mercuric oxide and zinc electrodes in an ...
is a non-rechargeable electrochemical battery, a primary cell
A primary battery or primary cell is a battery (a galvanic cell) that is designed to be used once and discarded, and not recharged with electricity and reused like a secondary cell (rechargeable battery). In general, the electrochemical reaction ...
, that was common in the middle of the 20th century. It was used in a wide variety of applications and was available in various sizes, particularly button sizes. Its constant voltage output and long shelf life gave it a niche use for camera light meters and hearing aids. The mercury cell was effectively banned in most countries in the 1990s due to concerns about the mercury contaminating landfills.
* Mercury was used for preserving wood, developing daguerreotype
Daguerreotype (; french: daguerréotype) was the first publicly available photographic process; it was widely used during the 1840s and 1850s. "Daguerreotype" also refers to an image created through this process.
Invented by Louis Daguerre an ...
s, silvering
Silvering is the chemical process of coating a non-conductive substrate such as glass with a reflective substance, to produce a mirror. While the metal is often silver, the term is used for the application of any reflective metal.
Process
Mos ...
mirror
A mirror or looking glass is an object that Reflection (physics), reflects an image. Light that bounces off a mirror will show an image of whatever is in front of it, when focused through the lens of the eye or a camera. Mirrors reverse the ...
s, anti-fouling paint
Anti-fouling paint is a specialized category of coatings applied as the outer (outboard) layer to the hull of a ship or boat, to slow the growth of and facilitate detachment of subaquatic organisms that attach to the hull and can affect a vess ...
s (discontinued in 1990), herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page fo ...
s (discontinued in 1995), interior latex paint, handheld maze games, cleaning, and road leveling devices in cars. Mercury compounds have been used in antiseptic
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putre ...
s, laxatives, antidepressant
Antidepressants are a class of medication used to treat major depressive disorder, anxiety disorders, chronic pain conditions, and to help manage addictions. Common side-effects of antidepressants include dry mouth, weight gain, dizziness, hea ...
s, and in antisyphilitics.
* It was allegedly used by allied spies to sabotage Luftwaffe planes: a mercury paste was applied to bare aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, causing the metal to rapidly corrode
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engin ...
; this would cause structural failures.
* Chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are comm ...
: The largest industrial use of mercury during the 20th century was in electrolysis for separating chlorine and sodium from brine; mercury being the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
of the Castner-Kellner process. The chlorine was used for bleaching paper (hence the location of many of these plants near paper mills) while the sodium was used to make sodium hydroxide for soaps and other cleaning products. This usage has largely been discontinued, replaced with other technologies that utilize membrane cells.
* As electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials de ...
s in some types of electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
, batteries ( mercury cells), sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
and chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
production, handheld games, catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s, insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to b ...
s.
* Mercury was once used as a gun barrel bore cleaner.
* From the mid-18th to the mid-19th centuries, a process called " carroting" was used in the making of felt
Felt is a textile material that is produced by matting, condensing and pressing fibers together. Felt can be made of natural fibers such as wool or animal fur, or from synthetic fibers such as petroleum-based acrylic or acrylonitrile or wood ...
hats. Animal skins were rinsed in an orange solution (the term "carroting" arose from this color) of the mercury compound mercuric nitrate, Hg(NO3)2·2H2O. This process separated the fur from the pelt and matted it together. This solution and the vapors it produced were highly toxic. The United States Public Health Service
The United States Public Health Service (USPHS or PHS) is a collection of agencies of the Department of Health and Human Services concerned with public health, containing nine out of the department's twelve operating divisions. The Assistant S ...
banned the use of mercury in the felt industry in December 1941. The psychological symptoms associated with mercury poisoning inspired the phrase "mad as a hatter
"Mad as a hatter" is a colloquial English phrase used in conversation to suggest (lightheartedly) that a person is suffering from insanity. The etymology of the phrase is uncertain, with explanations both connected and unconnected to the trade of ...
". Lewis Carroll
Charles Lutwidge Dodgson (; 27 January 1832 – 14 January 1898), better known by his pen name Lewis Carroll, was an English author, poet and mathematician. His most notable works are ''Alice's Adventures in Wonderland'' (1865) and its sequel ...
's "Mad Hatter
The Hatter is a fictional character in Lewis Carroll's 1865 book '' Alice's Adventures in Wonderland'' and its 1871 sequel ''Through the Looking-Glass''. He is very often referred to as the Mad Hatter, though this term was never used by Ca ...
" in his book ''Alice's Adventures in Wonderland
''Alice's Adventures in Wonderland'' (commonly ''Alice in Wonderland'') is an 1865 English novel by Lewis Carroll. It details the story of a young girl named Alice (Alice's Adventures in Wonderland), Alice who falls through a rabbit hole into a ...
'' was a play on words based on the older phrase, but the character himself does not exhibit symptoms of mercury poisoning.
* Gold and silver mining. Historically, mercury was used extensively in hydraulic gold mining in order to help the gold to sink through the flowing water-gravel mixture. Thin gold particles may form mercury-gold amalgam and therefore increase the gold recovery rates. Large-scale use of mercury stopped in the 1960s. However, mercury is still used in small scale, often clandestine, gold prospecting. It is estimated that 45,000 metric tons of mercury used in California for placer mining
Placer mining () is the mining of stream bed (Alluvium, alluvial) deposits for minerals. This may be done by open-pit mining, open-pit (also called open-cast mining) or by various surface excavating equipment or tunneling equipment.
Placer minin ...
have not been recovered. Mercury was also used in silver mining.
Historic medicinal uses
Mercury(I) chloride
Mercury(I) chloride is the chemical compound with the formula Hg2Cl2. Also known as the mineral calomel (a rare mineral) or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury(I) compound. ...
(also known as calomel or mercurous chloride) has been used in traditional medicine
Traditional medicine (also known as indigenous medicine or folk medicine) comprises medical aspects of traditional knowledge that developed over generations within the folk beliefs of various societies, including indigenous peoples, before the ...
as a diuretic
A diuretic () is any substance that promotes diuresis, the increased production of urine. This includes forced diuresis. A diuretic tablet is sometimes colloquially called a water tablet. There are several categories of diuretics. All diuretics in ...
, topical disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than st ...
, and laxative
Laxatives, purgatives, or aperients are substances that loosen stools and increase bowel movements. They are used to treat and prevent constipation.
Laxatives vary as to how they work and the side effects they may have. Certain stimulant, lubri ...
. Mercury(II) chloride
Mercury(II) chloride (or mercury bichloride, mercury dichloride), historically also known as sulema or corrosive sublimate, is the inorganic chemical compound of mercury and chlorine with the formula HgCl2. It is white crystalline solid and is ...
(also known as mercuric chloride or corrosive sublimate) was once used to treat syphilis
Syphilis () is a sexually transmitted infection caused by the bacterium ''Treponema pallidum'' subspecies ''pallidum''. The signs and symptoms of syphilis vary depending in which of the four stages it presents (primary, secondary, latent, an ...
(along with other mercury compounds), although it is so toxic that sometimes the symptoms of its toxicity were confused with those of the syphilis it was believed to treat. It is also used as a disinfectant. Blue mass
Blue mass (also known as blue pill or ''pilula hydrargyri'') was the name of a Mercury (element), mercury-based medicine common from the 17th to the 19th centuries. The oldest formula is ascribed to one Hayreddin Barbarossa, Barbarossa, in a lett ...
, a pill or syrup in which mercury is the main ingredient, was prescribed throughout the 19th century for numerous conditions including constipation, depression, child-bearing and toothaches. In the early 20th century, mercury was administered to children yearly as a laxative and dewormer, and it was used in teething powders for infants. The mercury-containing organohalide merbromin
Merbromin (marketed as Mercurochrome, Merbromine, Mercurocol, Sodium mercurescein, Asceptichrome, Supercrome, Brocasept and Cinfacromin) is an organomercuric disodium salt compound used as a topical antiseptic for minor cuts and scrapes and a ...
(sometimes sold as Mercurochrome) is still widely used but has been banned in some countries such as the U.S.
Toxicity and safety
Mercury and most of its compounds are extremely toxic and must be handled with care; in cases of spills involving mercury (such as from certainthermometers
A thermometer is a device that measures temperature or a temperature gradient (the degree of hotness or coldness of an object). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb of a mercury-in-glass thermomete ...
or fluorescent light bulb
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet ligh ...
s), specific cleaning procedures are used to avoid exposure and contain the spill. Protocols call for physically merging smaller droplets on hard surfaces, combining them into a single larger pool for easier removal with an eyedropper, or for gently pushing the spill into a disposable container. Vacuum cleaners and brooms cause greater dispersal of the mercury and should not be used. Afterwards, fine sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
, or some other powder that readily forms an amalgam (alloy) with mercury at ordinary temperatures is sprinkled over the area before itself being collected and properly disposed of. Cleaning porous surfaces and clothing is not effective at removing all traces of mercury and it is therefore advised to discard these kinds of items should they be exposed to a mercury spill.
Mercury can be absorbed through the skin and mucous membranes and mercury vapors can be inhaled, so containers of mercury are securely sealed to avoid spills and evaporation. Heating of mercury, or of compounds of mercury that may decompose when heated, should be carried out with adequate ventilation in order to minimize exposure to mercury vapor. The most toxic forms of mercury are its organic compounds
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
, such as dimethylmercury
Dimethylmercury (( C H3)2 Hg) is an extremely toxic organomercury compound. A highly volatile, reactive, flammable, and colorless liquid, dimethylmercury is one of the strongest known neurotoxins, with a quantity of less than 0.1 mL capable of in ...
and methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a ...
. Mercury can cause both chronic and acute poisoning.
Releases in the environment
volcano
A volcano is a rupture in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often found where tectonic plates are ...
es, are responsible for approximately half of atmospheric mercury emissions. The human-generated half can be divided into the following estimated percentages:
* 65% from stationary combustion, of which coal-fired power plant
A coal-fired power station or coal power plant is a thermal power station which burns coal to generate electricity. Worldwide, there are about 8,500 coal-fired power stations totaling over 2,000 gigawatts capacity. They generate about a th ...
s are the largest aggregate source (40% of U.S. mercury emissions in 1999). This includes power plants fueled with gas where the mercury has not been removed. Emissions from coal combustion are between one and two orders of magnitude higher than emissions from oil combustion, depending on the country.
* 11% from gold production. The three largest point sources for mercury emissions in the U.S. are the three largest gold mines. Hydrogeochemical release of mercury from gold-mine tailings has been accounted as a significant source of atmospheric mercury in eastern Canada.
* 6.8% from non-ferrous metal
In metallurgy, non-ferrous metals are metals or alloys that do not contain iron (allotropes of iron, ferrite, and so on) in appreciable amounts.
Generally more costly than ferrous metals, non-ferrous metals are used because of desirable proper ...
production, typically smelter
Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including Silver mining#Ore processing, silver, iron-making, iron, copper extracti ...
s.
* 6.4% from cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel ( aggregate) together. Cement mix ...
production.
* 3.0% from waste disposal
Waste management or waste disposal includes the processes and actions required to manage waste from its inception to its final disposal.
This includes the collection, transport, treatment and disposal of waste, together with monitoring ...
, including municipal
A municipality is usually a single administrative division having corporate status and powers of self-government or jurisdiction as granted by national and regional laws to which it is subordinate.
The term ''municipality'' may also mean the go ...
and hazardous waste
Hazardous waste is waste that has substantial or potential threats to public health or the environment. Hazardous waste is a type of dangerous goods. They usually have one or more of the following hazardous traits: ignitability, reactivity, co ...
, crematoria
Cremation is a method of final disposition of a dead body through burning.
Cremation may serve as a funeral or post-funeral rite and as an alternative to burial. In some countries, including India and Nepal, cremation on an open-air pyre i ...
, and sewage sludge
Sewage sludge is the residual, semi-solid material that is produced as a by-product during sewage treatment of industrial or municipal wastewater. The term "septage" also refers to sludge from simple wastewater treatment but is connected to si ...
incineration.
* 3.0% from caustic soda
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alka ...
production.
* 1.4% from pig iron
Pig iron, also known as crude iron, is an intermediate product of the iron industry in the production of steel which is obtained by smelting iron ore in a blast furnace. Pig iron has a high carbon content, typically 3.8–4.7%, along with silic ...
and steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
production.
* 1.1% from mercury production, mainly for batteries.
* 2.0% from other sources.
The above percentages are estimates of the global human-caused mercury emissions in 2000, excluding biomass burning, an important source in some regions.
Recent atmospheric mercury contamination in outdoor urban air was measured at 0.01–0.02 μg/m3. A 2001 study measured mercury levels in 12 indoor sites chosen to represent a cross-section of building types, locations and ages in the New York area. This study found mercury concentrations significantly elevated over outdoor concentrations, at a range of 0.0065 – 0.523 μg/m3. The average was 0.069 μg/m3.
Artificial lakes, or reservoir
A reservoir (; from French ''réservoir'' ) is an enlarged lake behind a dam. Such a dam may be either artificial, built to store fresh water or it may be a natural formation.
Reservoirs can be created in a number of ways, including contro ...
s, may be contaminated with mercury due to the absorption by the water of mercury from submerged trees and soil.
For example, Williston Lake
Williston Lake is a reservoir created by the W. A. C. Bennett Dam and is located in the Northern Interior of British Columbia, Canada.
Geography
The lake fills the basin of the upper Peace River, backing into the Rocky Mountain Trench which is ...
in northern British Columbia, created by the damming of the Peace River
The Peace River (french: links=no, rivière de la Paix) is a river in Canada that originates in the Rocky Mountains of northern British Columbia and flows to the northeast through northern Alberta. The Peace River joins the Athabasca River in th ...
in 1968, is still sufficiently contaminated with mercury that it is inadvisable to consume fish from the lake. Permafrost
Permafrost is ground that continuously remains below 0 °C (32 °F) for two or more years, located on land or under the ocean. Most common in the Northern Hemisphere, around 15% of the Northern Hemisphere or 11% of the global surface ...
soils have accumulated mercury through atmospheric deposition, and permafrost thaw in cryospheric regions is also a mechanism of mercury release into lakes, rivers, and wetland
A wetland is a distinct ecosystem that is flooded or saturated by water, either permanently (for years or decades) or seasonally (for weeks or months). Flooding results in oxygen-free (anoxic) processes prevailing, especially in the soils. The ...
s.
Mercury also enters into the environment through the improper disposal (e.g., land filling, incineration) of certain products. Products containing mercury include: auto parts, batteries, fluorescent bulbs, medical products, thermometers, and thermostats. Due to health concerns (see below), toxics use reduction
Toxics use reduction is an approach to pollution prevention that targets and measures reductions in the upfront use of toxic materials. Toxics use reduction emphasizes the more preventive aspects of source reduction but, due to its emphasis on toxi ...
efforts are cutting back or eliminating mercury in such products. For example, the amount of mercury sold in thermostats in the United States decreased from 14.5 tons in 2004 to 3.9 tons in 2007.
Most thermometers now use pigmented alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
instead of mercury. Mercury thermometers are still occasionally used in the medical field because they are more accurate than alcohol thermometers, though both are commonly being replaced by electronic thermometers and less commonly by galinstan thermometers. Mercury thermometers are still widely used for certain scientific applications because of their greater accuracy and working range.
Historically, one of the largest releases was from the Colex plant, a lithium isotope separation plant at Oak Ridge, Tennessee. The plant operated in the 1950s and 1960s. Records are incomplete and unclear, but government commissions have estimated that some two million pounds of mercury are unaccounted for.
A serious industrial disaster
This article lists notable industrial disasters, which are disasters caused by industrial companies, either by accident, negligence or incompetence. They are a form of industrial accident where great damage, injury or loss of life are caused.
Ot ...
was the dumping of waste mercury compounds into Minamata
is a city located in Kumamoto Prefecture, Japan. It is on the west coast of Kyūshū and faces Amakusa islands. Minamata was established as a village in 1889, re-designated as a town in 1912 and grew into a city in 1949. As of March 2017, the ...
Bay, Japan, between 1932 and 1968. It is estimated that over 3,000 people suffered various deformities, severe mercury poisoning symptoms or death from what became known as Minamata disease
Minamata disease is a neurological disease caused by severe mercury poisoning. Signs and symptoms include ataxia, numbness in the hands and feet, general muscle weakness, loss of peripheral vision, and damage to hearing and speech. In extrem ...
.
The tobacco plant
''Nicotiana'' () is a genus of herbaceous plants and shrubs in the family Solanaceae, that is indigenous to the Americas, Australia, Southwestern Africa and the South Pacific. Various ''Nicotiana'' species, commonly referred to as tobacco plants ...
readily absorbs and accumulates heavy metals
upright=1.2, Crystals of osmium, a heavy metal nearly twice as dense as lead">lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead
Heavy metals are generally defined as ...
such as mercury from the surrounding soil into its leaves. These are subsequently inhaled during tobacco smoking
Tobacco smoking is the practice of burning tobacco and ingesting the resulting smoke. The smoke may be inhaled, as is done with cigarettes, or simply released from the mouth, as is generally done with pipes and cigars. The practice is believed ...
. While mercury is a constituent of tobacco smoke
Tobacco smoke is a sooty aerosol produced by the incomplete combustion of tobacco during the tobacco smoking, smoking of cigarettes and other tobacco products. Temperatures in burning cigarettes range from about 400 °C between puffs to abo ...
, studies have largely failed to discover a significant correlation between smoking and Hg uptake by humans compared to sources such as occupational exposure, fish consumption, and amalgam tooth fillings.
Sediment contamination
Sediments within large urban-industrialestuaries
An estuary is a partially enclosed coastal body of brackish water with one or more rivers or streams flowing into it, and with a free connection to the open sea. Estuaries form a transition zone between river environments and maritime environment ...
act as an important sink for point source and diffuse mercury pollution within catchments. A 2015 study of foreshore sediments from the Thames estuary
The Thames Estuary is where the River Thames meets the waters of the North Sea, in the south-east of Great Britain.
Limits
An estuary can be defined according to different criteria (e.g. tidal, geographical, navigational or in terms of salini ...
measured total mercury at 0.01 to 12.07 mg/kg with mean of 2.10 mg/kg and median of 0.85 mg/kg (n=351). The highest mercury concentrations were shown to occur in and around the city of London
London is the capital and largest city of England and the United Kingdom, with a population of just under 9 million. It stands on the River Thames in south-east England at the head of a estuary down to the North Sea, and has been a majo ...
in association with fine grain muds and high total organic carbon content. The strong affinity of mercury for carbon rich sediments has also been observed in salt marsh sediments of the River Mersey
The River Mersey () is in North West England. Its name derives from Old English and means "boundary river", possibly referring to its having been a border between the ancient kingdoms of Mercia and Northumbria. For centuries it has formed part ...
mean of 2 mg/kg up to 5 mg/kg. These concentrations are far higher than those shown in salt marsh river creek sediments of New Jersey and mangrove
A mangrove is a shrub or tree that grows in coastal saline water, saline or brackish water. The term is also used for tropical coastal vegetation consisting of such species. Mangroves are taxonomically diverse, as a result of convergent evoluti ...
s of Southern China which exhibit low mercury concentrations of about 0.2 mg/kg.
Occupational exposure
 Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The
Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of h ...
, OSHA
OSHA or Osha may refer to:
Work
* Occupational Safety and Health Administration, a federal agency of the United States that regulates workplace safety and health
* Occupational Safety and Health Act (United States) of 1970, a federal law in the Un ...
, and NIOSH
The National Institute for Occupational Safety and Health (NIOSH, ) is the United States federal agency responsible for conducting research and making recommendations for the prevention of work-related injury and illness. NIOSH is part of the C ...
all treat mercury as an occupational hazard, and have established specific occupational exposure limits. Environmental releases and disposal of mercury are regulated in the U.S. primarily by the United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it be ...
.
Fish
Fish
Fish are aquatic, craniate, gill-bearing animals that lack limbs with digits. Included in this definition are the living hagfish, lampreys, and cartilaginous and bony fish as well as various extinct related groups. Approximately 95% of li ...
and shellfish
Shellfish is a colloquial and fisheries term for exoskeleton-bearing aquatic invertebrates used as food, including various species of molluscs, crustaceans, and echinoderms. Although most kinds of shellfish are harvested from saltwater envir ...
have a natural tendency to concentrate mercury in their bodies, often in the form of methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a ...
, a highly toxic organic compound of mercury. Species of fish that are high on the food chain
A food chain is a linear network of links in a food web starting from producer organisms (such as grass or algae which produce their own food via photosynthesis) and ending at an apex predator species (like grizzly bears or killer whales), det ...
, such as shark
Sharks are a group of elasmobranch fish characterized by a cartilaginous skeleton, five to seven gill slits on the sides of the head, and pectoral fins that are not fused to the head. Modern sharks are classified within the clade Selachimo ...
, swordfish
Swordfish (''Xiphias gladius''), also known as broadbills in some countries, are large, highly migratory predatory fish characterized by a long, flat, pointed bill. They are a popular sport fish of the billfish category, though elusive. Swordfis ...
, king mackerel
The king mackerel (''Scomberomorus cavalla'') or kingfish, is a migratory species of mackerel of the western Atlantic Ocean and Gulf of Mexico. It is an important species to both the commercial and recreational fishing industries.
Description
T ...
, bluefin tuna Bluefin tuna is a common name used to refer to several species of tuna of the genus ''Thunnus
''Thunnus'' is a genus of ocean-dwelling, ray-finned bony fish from the mackerel family, Scombridae. More specifically, ''Thunnus'' is one of five ...
, albacore tuna
The albacore (''Thunnus alalunga''), known also as the longfin tuna, is a species of tuna of the order Perciformes. It is found in temperate and tropical waters across the globe in the epipelagic and mesopelagic zones. There are six distinct sto ...
, and tilefish
250px, Blue blanquillo, ''Malacanthus latovittatus''
Tilefishes are mostly small perciform marine fish comprising the family Malacanthidae. They are usually found in sandy areas, especially near coral reefs.
Commercial fisheries exist for th ...
contain higher concentrations of mercury than others. Because mercury and methylmercury are fat soluble, they primarily accumulate in the viscera
In biology, an organ is a collection of tissues joined in a structural unit to serve a common function. In the hierarchy of life, an organ lies between tissue and an organ system. Tissues are formed from same type cells to act together in a ...
, although they are also found throughout the muscle tissue. Mercury presence in fish muscles can be studied using non-lethal muscle Biopsy, biopsies. Mercury present in prey fish accumulates in the predator that consumes them. Since fish are less efficient at depurating than accumulating methylmercury, methylmercury concentrations in the fish tissue increase over time. Thus species that are high on the food chain
A food chain is a linear network of links in a food web starting from producer organisms (such as grass or algae which produce their own food via photosynthesis) and ending at an apex predator species (like grizzly bears or killer whales), det ...
amass body burdens of mercury that can be ten times higher than the species they consume. This process is called biomagnification. Mercury poisoning
Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type, dose, method, and duration of exposure. They may include muscle weakness, poor coordination, numbness in the hands and feet, skin rashe ...
happened this way in Minamata, Kumamoto, Minamata, Japan, now called Minamata disease
Minamata disease is a neurological disease caused by severe mercury poisoning. Signs and symptoms include ataxia, numbness in the hands and feet, general muscle weakness, loss of peripheral vision, and damage to hearing and speech. In extrem ...
.
Cosmetics
Some facial creams contain dangerous levels of mercury. Most contain comparatively non-toxic inorganic mercury, but products containing highly toxic organic mercury have been encountered.Effects and symptoms of mercury poisoning
Toxic effects include damage to the brain, kidneys and lungs. Mercury poisoning can result in several diseases, including acrodynia (pink disease), Hunter-Russell syndrome, andMinamata disease
Minamata disease is a neurological disease caused by severe mercury poisoning. Signs and symptoms include ataxia, numbness in the hands and feet, general muscle weakness, loss of peripheral vision, and damage to hearing and speech. In extrem ...
.
Symptoms typically include sensory impairment (vision, hearing, speech), disturbed sensation and a lack of coordination. The type and degree of symptoms exhibited depend upon the individual toxin, the dose, and the method and duration of exposure. Case–control studies have shown effects such as tremors, impaired cognitive skills, and sleep disturbance in workers with chronic exposure to mercury vapor even at low concentrations in the range 0.7–42 μg/m3. A study has shown that acute exposure (4–8 hours) to calculated elemental mercury levels of 1.1 to 44 mg/m3 resulted in chest pain, dyspnea, cough, hemoptysis, impairment of pulmonary function, and evidence of interstitial pneumonitis. Acute exposure to mercury vapor has been shown to result in profound central nervous system effects, including psychotic reactions characterized by delirium, hallucinations, and suicidal tendency. Occupational exposure has resulted in broad-ranging functional disturbance, including erethism, irritability, excitability, excessive shyness, and insomnia. With continuing exposure, a fine tremor develops and may escalate to violent muscular spasms. Tremor initially involves the hands and later spreads to the eyelids, lips, and tongue. Long-term, low-level exposure has been associated with more subtle symptoms of erethism, including fatigue, irritability, loss of memory, vivid dreams and depression.
Treatment
Research on the treatment of mercury poisoning is limited. Currently available drugs for acute mercurial poisoning include chelators N-acetyl-D, L-penicillamine (NAP), British Anti-Lewisite (BAL), 2,3-dimercapto-1-propanesulfonic acid (DMPS), and dimercaptosuccinic acid (DMSA). In one small study including 11 construction workers exposed to elemental mercury, patients were treated with DMSA and NAP. Chelation therapy with both drugs resulted in the mobilization of a small fraction of the total estimated body mercury. DMSA was able to increase the excretion of mercury to a greater extent than NAP.Regulations
International
140 countries agreed in theMinamata Convention on Mercury
The Minamata Convention on Mercury is an international treaty designed to protect human health and the environment from anthropogenic emissions and releases of mercury and mercury compounds. The convention was a result of three years of meetin ...
by the United Nations Environment Programme (UNEP) to prevent emissions. The convention was signed on 10 October 2013.
United States
In the United States, the United States Environmental Protection Agency, Environmental Protection Agency is charged with regulating and managing mercury contamination. Several laws give the EPA this authority, including the Clean Air Act (United States), Clean Air Act, the Clean Water Act, the Resource Conservation and Recovery Act, and the Safe Drinking Water Act. Additionally, the Mercury-Containing and Rechargeable Battery Management Act, passed in 1996, phases out the use of mercury in batteries, and provides for the efficient and cost-effective disposal of many types of used batteries. North America contributed approximately 11% of the total global anthropogenic mercury emissions in 1995. The United States Clean Air Act (1990), Clean Air Act, passed in 1990, put mercury on a list of toxic pollutants that need to be controlled to the greatest possible extent. Thus, industries that release high concentrations of mercury into the environment agreed to install maximum achievable control technologies (MACT). In March 2005, the EPA promulgated a regulation that added power plants to the list of sources that should be controlled and instituted a national emissions trading, cap and trade system. States were given until November 2006 to impose stricter controls, but after a legal challenge from several states, the regulations were struck down by a federal appeals court on 8 February 2008. The rule was deemed not sufficient to protect the health of persons living near coal-fired power plants, given the negative effects documented in the EPA Study Report to Congress of 1998. However newer data published in 2015 showed that after introduction of the stricter controls mercury declined sharply, indicating that the Clean Air Act had its intended impact. The EPA announced new rules forcoal-fired power plant
A coal-fired power station or coal power plant is a thermal power station which burns coal to generate electricity. Worldwide, there are about 8,500 coal-fired power stations totaling over 2,000 gigawatts capacity. They generate about a th ...
s on 22 December 2011. Cement kilns that burn hazardous waste are held to a looser standard than are standard hazardous waste
Hazardous waste is waste that has substantial or potential threats to public health or the environment. Hazardous waste is a type of dangerous goods. They usually have one or more of the following hazardous traits: ignitability, reactivity, co ...
incinerators in the United States, and as a result are a disproportionate source of mercury pollution.
European Union
In theEuropean Union
The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been des ...
, the directive on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (see Restriction of Hazardous Substances Directive, RoHS) bans mercury from certain electrical and electronic products, and limits the amount of mercury in other products to less than 1000 Parts per million, ppm. Article 4 Paragraph 1. e.g. "Member States shall ensure that, from July 1, 2006, new electrical and electronic equipment put on the market does not contain lead, mercury, cadmium, hexavalent chromium, polybrominated biphenyls (PBB) or polybrominated diphenyl ethers (PBDE)." There are restrictions for mercury concentration in packaging (the limit is 100 ppm for sum of mercury, lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
, hexavalent chromium and cadmium) and batteries (the limit is 5 ppm). In July 2007, the European Union also banned mercury in non-electrical measuring devices, such as thermometer
A thermometer is a device that temperature measurement, measures temperature or a temperature gradient (the degree of hotness or coldness of an object). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb of a merc ...
s and barometer
A barometer is a scientific instrument that is used to measure air pressure in a certain environment. Pressure tendency can forecast short term changes in the weather. Many measurements of air pressure are used within surface weather analysis ...
s. The ban applies to new devices only, and contains exemptions for the health care sector and a two-year grace period for manufacturers of barometers.
Norway
Norway enacted a total ban on the use of mercury in the manufacturing and import/export of mercury products, effective 1 January 2008. In 2002, several lakes in Norway were found to have a poor state of mercury pollution, with an excess of 1 μg/g of mercury in their sediment. In 2008, Norway's Minister of Environment Development Erik Solheim said: "Mercury is among the most dangerous environmental toxins. Satisfactory alternatives to Hg in products are available, and it is therefore fitting to induce a ban."Sweden
Products containing mercury were banned in Sweden in 2009.Denmark
In 2008, Denmark also banned dental mercury amalgam, except for Molar (tooth), molar masticating surface fillings in permanent (adult) teeth.See also
* Mercury pollution in the ocean * Red mercury * COLEX process (isotopic separation)References
Further reading
*External links
Chemistry in its element podcast
(MP3) from the Royal Society of Chemistry's Chemistry World
Mercury
at ''The Periodic Table of Videos'' (University of Nottingham)
Centers for Disease Control and Prevention – Mercury Topic
Stopping Pollution: Mercury
– Oceana
Natural Resources Defense Council (NRDC): Mercury Contamination in Fish guide
– Natural Resources Defense Council, NRDC
NLM Hazardous Substances Databank – Mercury
BBC – Earth News – Mercury "turns" wetland birds such as ibises homosexual
Changing Patterns in the Use, Recycling, and Material Substitution of Mercury in the United States
United States Geological Survey
Thermodynamical data on liquid mercury.
* {{DEFAULTSORT:Mercury Mercury (element), Chemical elements Coolants Endocrine disruptors Native element minerals Neurotoxins Nuclear reactor coolants Occupational safety and health Transition metals