|
Mercuric Sulfate
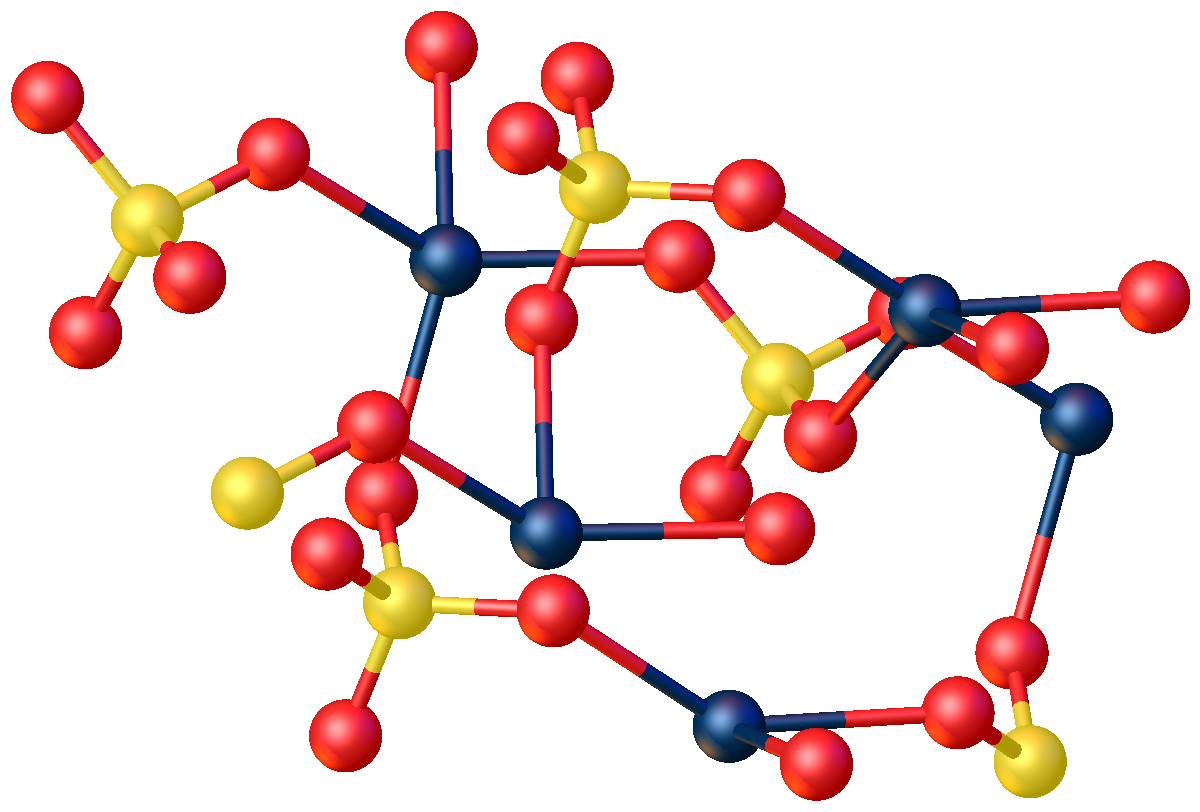
Mercury(II) sulfate, commonly called mercuric sulfate, is the chemical compound Hg S O4. It is an odorless solid that forms white granules or crystalline powder. In water, it separates into an insoluble sulfate with a yellow color and sulfuric acid. Structure The anhydrous compound features Hg2+ in a highly distorted tetrahedral HgO4 environment. Two Hg-O distances are 2.22 Å and the others are 2.28 and 2.42 Å. In the monohydrate, Hg2+ adopts a linear coordination geometry with Hg-O (sulfate) and Hg-O (water) bond lengths of 2.179 and 2.228 Å, respectively. Four weaker bonds are also observed with Hg---O distances >2.5 Å. History In 1932, the Japanese chemical company Chisso Corporation began using mercury sulfate as the catalyst for the production of acetaldehyde from acetylene and water. Though it was unknown at the time, methylmercury is formed as side product of this reaction. Exposure and consumption of the mercury waste products, including methylmercury, that were ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
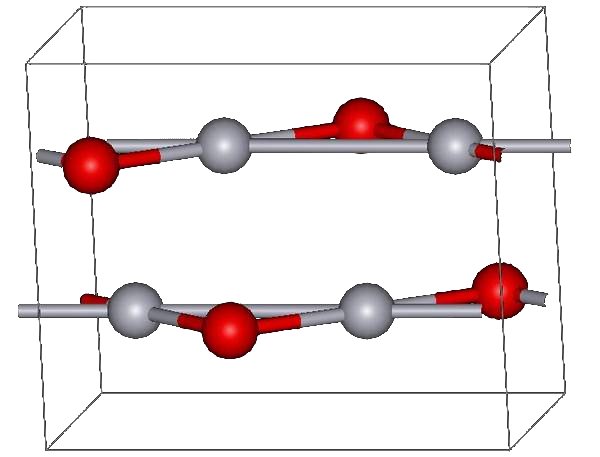
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal classes The table below ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolate potassium metal. By heating potassium carbonate with carbon at very high temperatures, he produce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Markovnikov's Rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870. Explanation The rule states that with the addition of a protic acid HX or other polar reagent to an asymmetric alkene, the acid hydrogen (H) or electropositive part gets attached to the carbon with more hydrogen substituents, and the halide (X) group or electronegative part gets attached to the carbon with more alkyl substituents. This is in contrast to Markovnikov's original definition, in which the rule is stated that the X component is added to the carbon with the fewest hydrogen atoms while the hydrogen atom is added to the carbon with the greatest number of hydrogen atoms. The same is true when an alkene reacts with water in an addition reaction to form an alcohol which involve formation of carbocations. The hydroxyl group (OH) bonds to the carbon that has the greater number of carbon– ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxymercuration Reaction
The oxymercuration reaction is an electrophilic addition organic reaction that transforms an alkene into a neutral alcohol. In oxymercuration, the alkene reacts with mercuric acetate (AcO–Hg–OAc) in aqueous solution to yield the addition of an acetoxymercury (HgOAc) group and a hydroxy (OH) group across the double bond. Carbocations are not formed in this process and thus rearrangements are not observed. The reaction follows Markovnikov's rule (the hydroxy group will always be added to the more substituted carbon) and it is an anti addition (the two groups will be trans to each other). Oxymercuration followed by reductive demercuration is called an oxymercuration–reduction reaction or oxymercuration–demercuration reaction. This reaction, which is almost always done in practice instead of oxymercuration, is treated at the conclusion of the article. Mechanism Oxymercuration can be fully described in three steps (the whole process is sometimes called ''deoxymercuration''), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Acetate
Mercury(II) acetate is the chemical compound with the formula Hg( O2 CC H3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white water-soluble solid, but samples appear yellowish with time owing to decomposition. Structure Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 Å. Three long, weak intermolecular Hg···O bonds of about 2.75 Å are also present, resulting in a slightly distorted square pyramidal coordination geometry at Hg. Synthesis and reactions Mercury(II) acetate can be produced by reaction of mercuric oxide with acetic acid. Inorganic reactions Mercury(II) acetate in acetic acid solution reacts with H2S to rapidly precipitate the black (β) polymorph of HgS. With gentle heating of the slurry, the black solid converts to the red form. The mineral cinnabar is red HgS. The precipita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Denigés' Reagent
The Denigés' reagent is a reagent used for qualitative analysis. It was developed in 1898 by Georges Denigés (December 25, 1859–February 20, 1951), a French biochemist. Uses Denigés' reagent is used to detect isolefin or tertiary alcohols which can be easily dehydrated to form isoolefin in the presence of acid. Treatment of solutions containing either isolefin or tertiary alcohols with this reagent will result in the formation of a solid yellow or red precipitate. Synthesis Despite the different stoichiometry in these mixtures which varies the concentration of the reagent, they all follow the same idea of adding HgO to distilled water and concentrated sulfuric acid. The Denigés' reagent is ultimately mercury(II) sulfate in an aqueous solution. *5 grams of mercury(II) oxide (HgO) is dissolved in 40 mL of distilled water. The mixture is slowly stirred, while 20 mL of concentrated sulfuric acid is added. After adding another 40 mL of distilled water, the solution is s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercuric Oxide
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is very rarely found. History An experiment for the preparation of mercuric oxide was first described by 11th century Arab-Spanish alchemist, Maslama al-Majriti, in ''Rutbat al-hakim.'' In 1774, Joseph Priestley discovered that oxygen was released by heating mercuric oxide, although he did not identify the gas as oxygen (rather, Priestley called it " dephlogisticated air," as that was the paradigm that he was working under at the time). Synthesis The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by pyrolysis of Hg(NO3)2. The yellow form can be obtained by precipitation of aqueous Hg2+ with alkali. The difference in color is due to particle size; both forms have the same structure consisting of near lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north toward the East China Sea, Philippine Sea, and Taiwan in the south. Japan is a part of the Ring of Fire, and spans an archipelago of 6852 islands covering ; the five main islands are Hokkaido, Honshu (the "mainland"), Shikoku, Kyushu, and Okinawa. Tokyo is the nation's capital and largest city, followed by Yokohama, Osaka, Nagoya, Sapporo, Fukuoka, Kobe, and Kyoto. Japan is the eleventh most populous country in the world, as well as one of the most densely populated and urbanized. About three-fourths of the country's terrain is mountainous, concentrating its population of 123.2 million on narrow coastal plains. Japan is divided into 47 administrative prefectures and eight traditional regions. The Greater Tokyo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minamata, Kumamoto
is a city located in Kumamoto Prefecture, Japan. It is on the west coast of Kyūshū and faces Amakusa islands. Minamata was established as a village in 1889, re-designated as a town in 1912 and grew into a city in 1949. As of March 2017, the city has an estimated population of 25,310 and a population density of 160 persons per km². The total area is 162.88 km². Minamata is known due to Minamata disease, a neurological disorder caused by mercury poisoning. The disease was discovered in 1956. A local chemical plant was blamed for causing the disease by emitting untreated wastewater into Minamata Bay. Lately, Minamata has focused on becoming a model environmental city. In 1999, the city obtained the ISO 14001 certification for Environmental Management. In 2001, Minamata became an official Japanese Eco-town. In 2004 and 2005, Minamata won the Japanese Top Eco-City contest. Minamata environmental disaster History The city is best known as the former site of an environ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minamata Disease
Minamata disease is a neurological disease caused by severe mercury poisoning. Signs and symptoms include ataxia, numbness in the hands and feet, general muscle weakness, loss of peripheral vision, and damage to hearing and speech. In extreme cases, insanity, paralysis, coma, and death follow within weeks of the onset of symptoms. A congenital form of the disease can also affect fetuses in the womb and may cause cerebral palsy. Minamata disease was first discovered in the city of Minamata, Kumamoto Prefecture, Japan, in 1956, hence its name. It was caused by the release of methylmercury in the industrial wastewater from a chemical factory owned by the Chisso Corporation, which continued from 1932 to 1968. It has also been suggested that some of the mercury sulfate in the wastewater was also metabolized to methylmercury by bacteria in the sediment. This highly toxic chemical bioaccumulated and biomagnified in shellfish and fish in Minamata Bay and the Shiranui Sea, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a bioaccumulative environmental toxicant. Structure and chemistry "Methylmercury" is a shorthand for the hypothetical "methylmercury cation", sometimes written "''methylmercury(1+) cation''" or "''methylmercury(II) cation''". This functional group is composed of a methyl group bonded to an atom of mercury. Its chemical formula is (sometimes written as ).The Methylmercury compound has an overall charge of +1, with Hg in the +2 oxidation state.Methylmercury exists as a substituent in many complexes of the type (L = Lewis base) and MeHgX (X = anion). As a positively charged ion it readily combines with anions such as chloride (), hydroxide () and nitrate (). It has particular affinity for sulfur-containing anions, particularly th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |