MFSD6L Predicted Tertiary Structure on:
[Wikipedia]
[Google]
[Amazon]
 Major facilitator superfamily domain containing 6 like (MFSD6L) is a
Major facilitator superfamily domain containing 6 like (MFSD6L) is a
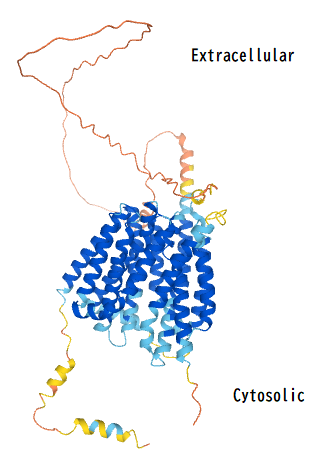
 The peptide sequence contains 11 transmembrane regions that cross the plasma membrane. Additionally, there are also two MFS regions starting at the 28th and 368th encoding amino acids.
For the secondary structure of the MFSD6L protein, there are 16 predicted alpha helices and 3 predicted beta sheets. The large amount of alpha helices within the structure of MFSD6L can be attributed to the protein being a transmembrane solute transporter since alpha helices are usually the part of the protein's structure that is positioned within the cell membrane.
Within the tertiary structure, there was a disulfide bond predicted between the two cysteines at the 29th and 311th amino acids.
The peptide sequence contains 11 transmembrane regions that cross the plasma membrane. Additionally, there are also two MFS regions starting at the 28th and 368th encoding amino acids.
For the secondary structure of the MFSD6L protein, there are 16 predicted alpha helices and 3 predicted beta sheets. The large amount of alpha helices within the structure of MFSD6L can be attributed to the protein being a transmembrane solute transporter since alpha helices are usually the part of the protein's structure that is positioned within the cell membrane.
Within the tertiary structure, there was a disulfide bond predicted between the two cysteines at the 29th and 311th amino acids.
 There was only one promoter region, spanning 1,107 bp, found for MFSD6L using the Genomatix Gene2Promoter database. For the part of the promoter region closest to the start of the 5' UTR of the MFSD6L gene, there were several transcription factor binding sites found. A transcription factor binding site of note was the site for the p53 tumor suppressor protein.
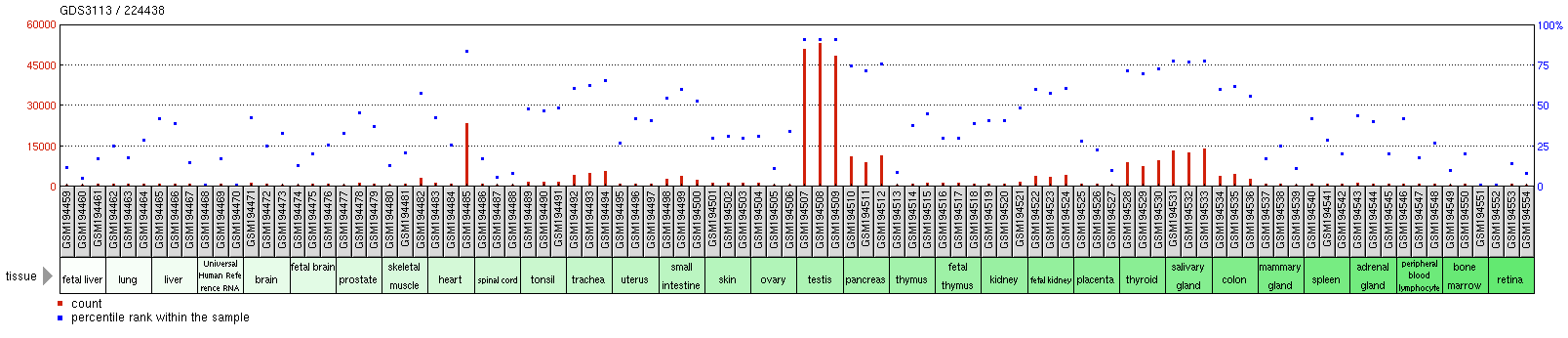
The MFSD6L gene was found to be highly expressed in the pancreas, salivary glands, and the thyroid.
Inspection of in-situ hybridization expression of MFSD6L gene shows that the gene is particularly expressed within glandular cells within their respective tissues.
Expression of the MFSD6L was found to be upregulated as a result of glucose starvation.
There was only one promoter region, spanning 1,107 bp, found for MFSD6L using the Genomatix Gene2Promoter database. For the part of the promoter region closest to the start of the 5' UTR of the MFSD6L gene, there were several transcription factor binding sites found. A transcription factor binding site of note was the site for the p53 tumor suppressor protein.
The MFSD6L gene was found to be highly expressed in the pancreas, salivary glands, and the thyroid.
Inspection of in-situ hybridization expression of MFSD6L gene shows that the gene is particularly expressed within glandular cells within their respective tissues.
Expression of the MFSD6L was found to be upregulated as a result of glucose starvation.


 Major facilitator superfamily domain containing 6 like (MFSD6L) is a
Major facilitator superfamily domain containing 6 like (MFSD6L) is a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
encoded by the MFSD6L gene in humans. The MFSD6L protein is a transmembrane protein that is part of the major facilitator superfamily (MFS) that uses chemiosmotic gradients to facilitate the transport of small solutes across cell membranes.
Gene
In the human genome, the MFSD6L gene is located on chromosome 17 (17p13.1). The DNA sequence encoding the polypeptide encompasses 2,256 bases, starting from 8,797,110 bp to 8,799,365 bp. Additionally, the gene sequence resides on the minus strand. The MFSD6L gene has one alias called FLJ35773. The encoding DNA sequence results in only one exon in the translated mRNA sequence. Thetumor suppressor gene
A tumor suppressor gene (TSG), or anti-oncogene, is a gene that regulates a cell during cell division and replication. If the cell grows uncontrollably, it will result in cancer. When a tumor suppressor gene is mutated, it results in a loss or red ...
TP53 was also found within the gene neighborhood of MFSD6L at 17p13.1.
mRNA Transcript
The MFSD6L gene was not found to have other isoforms due to the presence of only one exon in the MFSD6L encoding sequence.Protein
The MFSD6L protein has a precursormolecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
of approximately 64 kDa, consisting of 586 amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
s. After post-translational modifications, such as glycosylation, the mature MFSD6L protein's molecular weight increases to 72 kDa. Of the amino acids consisting the MFSD6L protein, leucine was found to have increased levels compared to most other human proteins. This increase in leucine is also present in the MFSD6L protein of the house mouse
The house mouse (''Mus musculus'') is a small mammal of the order Rodentia, characteristically having a pointed snout, large rounded ears, and a long and almost hairless tail. It is one of the most abundant species of the genus '' Mus''. Althoug ...
and chimpanzee
The chimpanzee (''Pan troglodytes''), also known as simply the chimp, is a species of great ape native to the forest and savannah of tropical Africa. It has four confirmed subspecies and a fifth proposed subspecies. When its close relative th ...
. The protein also has an isoelectric point
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also u ...
of 8.87 pI.
 The peptide sequence contains 11 transmembrane regions that cross the plasma membrane. Additionally, there are also two MFS regions starting at the 28th and 368th encoding amino acids.
For the secondary structure of the MFSD6L protein, there are 16 predicted alpha helices and 3 predicted beta sheets. The large amount of alpha helices within the structure of MFSD6L can be attributed to the protein being a transmembrane solute transporter since alpha helices are usually the part of the protein's structure that is positioned within the cell membrane.
Within the tertiary structure, there was a disulfide bond predicted between the two cysteines at the 29th and 311th amino acids.
The peptide sequence contains 11 transmembrane regions that cross the plasma membrane. Additionally, there are also two MFS regions starting at the 28th and 368th encoding amino acids.
For the secondary structure of the MFSD6L protein, there are 16 predicted alpha helices and 3 predicted beta sheets. The large amount of alpha helices within the structure of MFSD6L can be attributed to the protein being a transmembrane solute transporter since alpha helices are usually the part of the protein's structure that is positioned within the cell membrane.
Within the tertiary structure, there was a disulfide bond predicted between the two cysteines at the 29th and 311th amino acids.
Expression and regulation
Gene level
 There was only one promoter region, spanning 1,107 bp, found for MFSD6L using the Genomatix Gene2Promoter database. For the part of the promoter region closest to the start of the 5' UTR of the MFSD6L gene, there were several transcription factor binding sites found. A transcription factor binding site of note was the site for the p53 tumor suppressor protein.
The MFSD6L gene was found to be highly expressed in the pancreas, salivary glands, and the thyroid.
Inspection of in-situ hybridization expression of MFSD6L gene shows that the gene is particularly expressed within glandular cells within their respective tissues.
Expression of the MFSD6L was found to be upregulated as a result of glucose starvation.
There was only one promoter region, spanning 1,107 bp, found for MFSD6L using the Genomatix Gene2Promoter database. For the part of the promoter region closest to the start of the 5' UTR of the MFSD6L gene, there were several transcription factor binding sites found. A transcription factor binding site of note was the site for the p53 tumor suppressor protein.
The MFSD6L gene was found to be highly expressed in the pancreas, salivary glands, and the thyroid.
Inspection of in-situ hybridization expression of MFSD6L gene shows that the gene is particularly expressed within glandular cells within their respective tissues.
Expression of the MFSD6L was found to be upregulated as a result of glucose starvation.

Transcript level
Since there is only one exon and no introns within the MFSD6L gene, There is no splicing performed on the MFSD6L mRNA. Translation of the MFSD6L protein initiates at the end of the 5' UTR, which is the first 245 nucleotides of the MFSD6L mRNA. There are conserved stem-loop regions across mammalian orthologs, which infer possible miRNA binding sites.Protein level
The subcellular localization of the MFSD6L protein is predicted to be within the cell membrane via DeepLoc tool. This is supported by it being a solute symporter similar to MFS proteins. The first 28 amino acids of the translated MFSD6L protein contains the signal peptide. Additionally, n-glycosylation sites were predicted at the 110th, 129th, and 224th amino acids of the protein sequence. A serine phosphorylation site at the 429th amino acid was also predicted and verified by presence within other mammalian orthologs.Evolution
Paralogs
The MFSD6 protein was found to be the only paralog to the human MFSD6L protein.Orthologs
Through BLAST sequence analysis, the MFSD6L protein was found to have orthologs in a many mammalian species, especially amongprimate
Primates are a diverse order of mammals. They are divided into the strepsirrhines, which include the lemurs, galagos, and lorisids, and the haplorhines, which include the tarsiers and the simians (monkeys and apes, the latter including huma ...
s, and flying foxes. There were some orthologs found in the Reptilia and Amphibia
Amphibians are four-limbed and ectothermic vertebrates of the class Amphibia. All living amphibians belong to the group Lissamphibia. They inhabit a wide variety of habitats, with most species living within terrestrial, fossorial, arbore ...
classes, albeit not as great in number as in the Mammalia
Mammals () are a group of vertebrate animals constituting the class Mammalia (), characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur o ...
. Among fish, there were significantly more orthologs found amongst ray-finned
Actinopterygii (; ), members of which are known as ray-finned fishes, is a class of bony fish. They comprise over 50% of living vertebrate species.
The ray-finned fishes are so called because their fins are webs of skin supported by bony or hor ...
fishes than cartilaginous fishes. Additionally, the jaw-less fish, the sea lamprey, was also found to be an ortholog.
There were also multiple orthologs found amongst invertebrates, such as echinoderms and mollusks.
No significant orthologs of the MFSD6L protein were found amongst insect
Insects (from Latin ') are pancrustacean hexapod invertebrates of the class Insecta. They are the largest group within the arthropod phylum. Insects have a chitinous exoskeleton, a three-part body ( head, thorax and abdomen), three pairs ...
s; however there were orthologs found in the bacteria. Specifically, the Anaerolinea
''Anaerolinea'' is a bacteria genus from the family of Anaerolineaceae
Anaerolineaceae is a family of bacteria from the order of Anaerolineales.
Anaerolineaceae bacteria occur in marine sediments. There are a total of twelve genera in this fa ...
genus, which contains thermophilic bacteria were found to have orthologs with the human protein due to its regions of MFS being identical to MFS regions found in the human protein. The following table shows some examples of orthologs of the human MFSD6L.
Homologous gomains
The main homologous domains found within the MFSD6L protein are the MFS regions. Since MFS includes a large amount of solute transporter proteins within its superfamily, there are many MFS proteins that have the same homologous MFS domains.Most distant homologs
Through BLAST sequence analysis, the most distant homologs were the organisms within theCnidaria
Cnidaria () is a phylum under kingdom Animalia containing over 11,000 species of aquatic animals found both in freshwater and marine environments, predominantly the latter.
Their distinguishing feature is cnidocytes, specialized cells that th ...
phylum
In biology, a phylum (; plural: phyla) is a level of classification or taxonomic rank below kingdom and above class. Traditionally, in botany the term division has been used instead of phylum, although the International Code of Nomenclature f ...
, which mainly consists of jellyfish, sea anemones, and corals. Searching with BLAST for the MFSD6L gene at an older diverging phylum, the Porifera
Sponges, the members of the phylum Porifera (; meaning 'pore bearer'), are a basal animal clade as a sister of the diploblasts. They are multicellular organisms that have bodies full of pores and channels allowing water to circulate through th ...
, revealed no homologous MFSD6L protein.
Predicted emergence date
As a result of the MFSD6L protein's presence in Cnidaria and absence in Porifera, the estimated emergence date of the MFSD6L gene lies between 687 and 777 MYA, which are the divergence dates found from TimeTree. From the corrected % divergence chart and calculations of the corrected % divergence of the ''Homo sapiens'' MFSD6 paralog, the estimated date of emergence of the MFSD6L protein was found to be around 736 MYA.Interacting proteins
The MFSD6L protein was not found to have any experimentally-verified protein-protein interactions.Function
The polypeptide sequence contains many transmembrane regions, identifying the MFSD6L protein as a transmembrane protein for transporting solutes across the plasma membrane of a cell. Tertiary structure prediction tools suggest that the structure of the MFSD6L protein is similar to 1PV6A, a β-galactosidessymporter
A symporter is an integral membrane protein that is involved in the transport of two (or more) different molecules across the cell membrane in the same direction. The symporter works in the plasma membrane and molecules are transported across the ...
which uses proton gradients to transport solutes. As a result, the function of the MFSD6L protein could possibly a sugar symporter. This is additionally supported by the fact that the expression of MFSD6L was upregulated due to glucose starvation.
Clinical significance
Disease association
A disease associated with the MFSD6L gene is the Tetralogy of Fallot, which is a series of four congenital heart defects that can cause low oxygenation of blood. This is due to a ventricular septal defect that causes the mixing of oxygenated and deoxygenated blood in the left ventricle of the heart. The MFSD6L gene was also found to be a candidate gene taking part in the disease Pediatric Cataract. 602x602px, A corrected % divergence chart of the MFSD6L protein. Cytochrome C and Fibrinogen Alpha Chain is also added for comparison.Mutations
Various SNP's were found within the encoding sequence of the MFSD6L protein sequence as shown below.References
{{Reflist