Ladderane Nomenclature on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 Synthetic approaches have yielded ladderanes of varying lengths. A classification system has been developed to describe ladderanes based on the number of consecutive rings. The length of the ladderane is described by the number in brackets that precedes the word "ladderane". This is equal to the number of bonds shared by two cyclobutanes (''n'') plus 1.
A ladderane of 3 or more units can connect in a circle, forming a band, which can also be considered to be two interconnected parallel
Synthetic approaches have yielded ladderanes of varying lengths. A classification system has been developed to describe ladderanes based on the number of consecutive rings. The length of the ladderane is described by the number in brackets that precedes the word "ladderane". This is equal to the number of bonds shared by two cyclobutanes (''n'') plus 1.
A ladderane of 3 or more units can connect in a circle, forming a band, which can also be considered to be two interconnected parallel
 Ladderanes have two types of stereochemical relationships. One describes the relative arrangement of
Ladderanes have two types of stereochemical relationships. One describes the relative arrangement of
A different synthetic approach developed by Martin and coworkers has allowed for the synthesis of ladderanes. The initial step involves the formation of a ladderane from the addition of two equivalents of maleic anhydride with 

 MacGillivray and colleagues have demonstrated that a supramolecular approach to covalent synthesis in the organized, solvent-free environment of the solid state can provide a solution to the problem of organizing two polyenes for an intramolecular reaction to give a ladderane. Specifically, by taking an approach to control reactivity in solids by using molecules that serve as linear templates, they have demonstrated the utility of cocrystallization of resorcinol (1,3-benzenediol), or a derivative, with an all-''trans''-bis(4-pyridyl)poly-''m''-ene (4-pyr-poly-''m''-ene) produces a four-component molecular assembly, 2(resorcinol)·2(4-pyr-poly-''m''-ene), in which each resorcinol preorganizes, through two O—H···N hydrogen-bonding interactions, two poly-''m''-enes for +2photoaddition. The two polyenes are positioned by the templates such that the C=C bonds of the olefins lie parallel and separated by < 4.2 Å, a position suitable for the photoreaction. UV irradiation of the solid produces the targeted 'n''adderane, with the C=C bonds reacting to form the fused cyclobutane framework. Broadband UV-irradiation of two such hydrogen-bonded, four-component supramolecular assemblies furnishes the corresponding ladderanes stereospecifically and in quantitative yield in gram quantities.
MacGillivray and colleagues have demonstrated that a supramolecular approach to covalent synthesis in the organized, solvent-free environment of the solid state can provide a solution to the problem of organizing two polyenes for an intramolecular reaction to give a ladderane. Specifically, by taking an approach to control reactivity in solids by using molecules that serve as linear templates, they have demonstrated the utility of cocrystallization of resorcinol (1,3-benzenediol), or a derivative, with an all-''trans''-bis(4-pyridyl)poly-''m''-ene (4-pyr-poly-''m''-ene) produces a four-component molecular assembly, 2(resorcinol)·2(4-pyr-poly-''m''-ene), in which each resorcinol preorganizes, through two O—H···N hydrogen-bonding interactions, two poly-''m''-enes for +2photoaddition. The two polyenes are positioned by the templates such that the C=C bonds of the olefins lie parallel and separated by < 4.2 Å, a position suitable for the photoreaction. UV irradiation of the solid produces the targeted 'n''adderane, with the C=C bonds reacting to form the fused cyclobutane framework. Broadband UV-irradiation of two such hydrogen-bonded, four-component supramolecular assemblies furnishes the corresponding ladderanes stereospecifically and in quantitative yield in gram quantities.
 Ladderanes were first identified in a rare group of anaerobic ammonium oxidizing ( anammox) bacteria belonging to the phylum Planctomycetota. These bacteria sequester the catabolic anammox reactions to intracellular compartments called anammoxosomes. The anammox process involves the oxidation of
Ladderanes were first identified in a rare group of anaerobic ammonium oxidizing ( anammox) bacteria belonging to the phylum Planctomycetota. These bacteria sequester the catabolic anammox reactions to intracellular compartments called anammoxosomes. The anammox process involves the oxidation of
 In 2016, Burns and co-workers at Stanford University reported an enantioselective synthesis of both the and ladderane lipid tails and their incorporation into a full phosphatidylcholine lipid.
Both routes leverage a small ladderene building block bicyclo .2.0exene prepared by a Ramberg–Bäcklund reaction. The route to a ladderane-containing fatty acid involves dimerization of this intermediate to form an all-''anti'' ladderane hydrocarbon. C–H chlorination by a manganese porphyrin catalyst and subsequent elimination introduces unsaturation to produce a ladderene. Hydroboration and a Zweifel reaction install the linear alkyl group.
In 2016, Burns and co-workers at Stanford University reported an enantioselective synthesis of both the and ladderane lipid tails and their incorporation into a full phosphatidylcholine lipid.
Both routes leverage a small ladderene building block bicyclo .2.0exene prepared by a Ramberg–Bäcklund reaction. The route to a ladderane-containing fatty acid involves dimerization of this intermediate to form an all-''anti'' ladderane hydrocarbon. C–H chlorination by a manganese porphyrin catalyst and subsequent elimination introduces unsaturation to produce a ladderene. Hydroboration and a Zweifel reaction install the linear alkyl group.
 The route to a ladderane fatty alcohol begins with a +2photocycloaddition between a brominated benzoquinone and bicyclo .2.0exene. Elimination of H–Br and addition of an organozinc compound installs the alkyl alcohol. A
The route to a ladderane fatty alcohol begins with a +2photocycloaddition between a brominated benzoquinone and bicyclo .2.0exene. Elimination of H–Br and addition of an organozinc compound installs the alkyl alcohol. A 
 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, a ladderane is an organic molecule containing two or more fused cyclobutane rings. The name arises from the resemblance of a series of fused cyclobutane rings to a ladder. Numerous synthetic approaches have been developed for the synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
of ladderane compounds of various lengths. The mechanisms often involve + 2photocycloadditions, a useful reaction for creating strained 4-membered rings. Naturally occurring ladderanes have been identified as major components of the anammoxosome membrane of the anammox bacteria, phylum '' Planctomycetota''.
Nomenclature
Chain length
 Synthetic approaches have yielded ladderanes of varying lengths. A classification system has been developed to describe ladderanes based on the number of consecutive rings. The length of the ladderane is described by the number in brackets that precedes the word "ladderane". This is equal to the number of bonds shared by two cyclobutanes (''n'') plus 1.
A ladderane of 3 or more units can connect in a circle, forming a band, which can also be considered to be two interconnected parallel
Synthetic approaches have yielded ladderanes of varying lengths. A classification system has been developed to describe ladderanes based on the number of consecutive rings. The length of the ladderane is described by the number in brackets that precedes the word "ladderane". This is equal to the number of bonds shared by two cyclobutanes (''n'') plus 1.
A ladderane of 3 or more units can connect in a circle, forming a band, which can also be considered to be two interconnected parallel cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing ...
rings. These are called prismanes.
Stereochemistry
 Ladderanes have two types of stereochemical relationships. One describes the relative arrangement of
Ladderanes have two types of stereochemical relationships. One describes the relative arrangement of hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen consti ...
s at the fusion between two cyclobutane rings. These hydrogen atoms can be in either the cis- or trans- configuration. Trans-ladderanes have not been synthesized due to the ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are su ...
in these compounds.
The second stereochemical relationship describes the orientation of three consecutive cyclobutane rings, and therefore is only relevant to ladderanes of ''n'' ≥ 2. The two outer rings can be on the same face (syn-) or on the opposite face (anti-) of the center ring.
Synthesis
Various synthetic methods have been used for the laboratory synthesis of ladderane compounds. The three major approaches are (1)dimerization
A dimer () (''wikt:di-, di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, Covalent bond, covalent or Intermolecular force, intermolecular. Dimers also have significant im ...
of polyene precursors, (2) the stepwise addition, one or two rings at a time, (3) and oligomerization. Several examples of ladderane synthesis are outlined below.
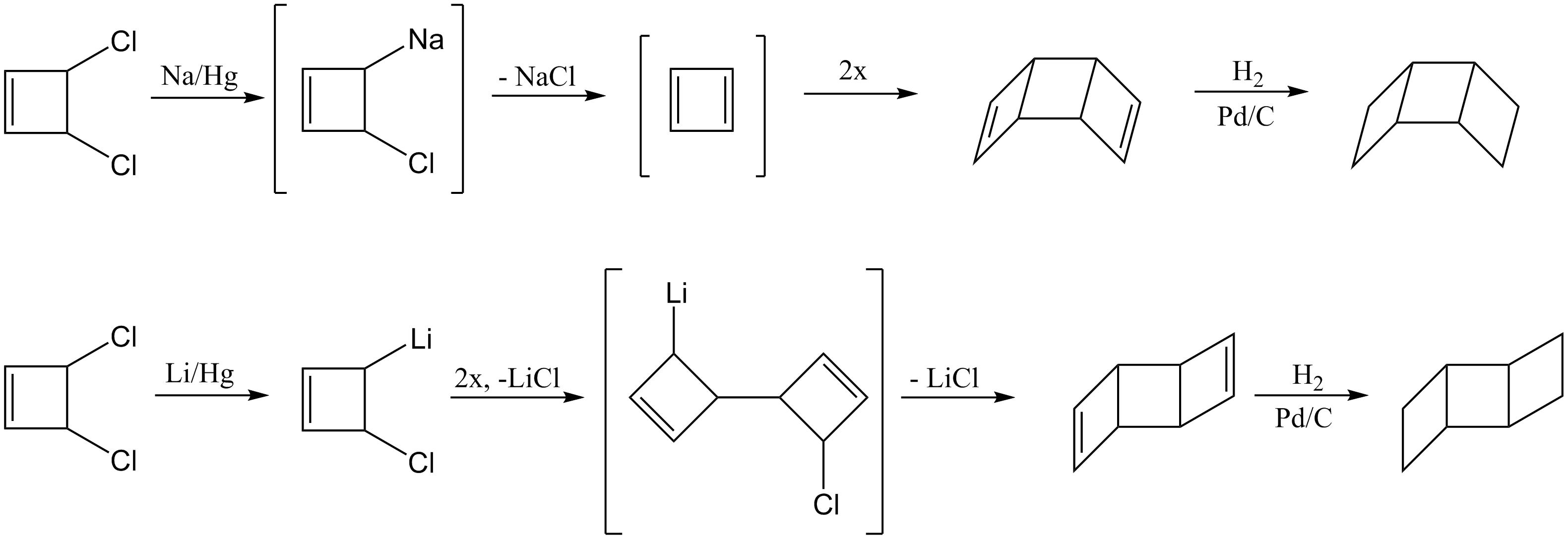
Dimerization of cyclobutadiene
The dimerization of two cyclobutadienes can generate both the syn and anti ladderane products depending on the reaction conditions. The first step in forming the syn product involves the generation of 1,3-cyclobutadiene by treatment of cis-3,4-dichlorocyclobutene with sodium amalgam. The reactant passes through a metalated intermediate before forming 1,3-cyclobutadiene, which can then dimerize to form the syn-diene. Hydrogenation of the double bonds will form the saturated syn- ladderane. To generate the anti product, cis-3,4-dichlorocyclobutene is treated with lithium amalgam. The lithium derivative undergoes a C-C coupling reaction to produce the open dimeric structure. This intermediate reacts to form the anti-diene, which can be hydrogenated to form the final anti- ladderane product.
acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
. The remaining two rings are formed from the Ramberg–Bäcklund ring contraction.

Synthesis of long-chain ladderanes
Ladderanes with lengths up to 13 cyclobutane rings have been synthesized by Mehta and coworkers. This process involves the ''in situ'' generation of dicarbomethoxycyclobutadiene from its Fe(CO)3 complex at low temperatures with the addition of ceric(IV) ammonium nitrate (CAN). Generation of the butadiene rapidly forms a mixture of 'n''ladderanes of lengths up to ''n'' = 13 with an overall yield of 55%. All of the ladderanes synthesized through this method have one cis,syn,cis structure. This may be a result of the initial dimerization of two cyclobutadienes which preferably forms the syn product, shown below. The further dimerization only produces the anti product due to steric factors.
Dimerization of polyene precursors
In these reactions, ladderanes are formed from multiple + 2photocycloadditions between the double bonds of two polyenes. A complication that arises from this approach is the reaction of the precursors through alternative, more favorable photoexcitation routes. These side reactions are prevented by the addition of a chemical spacer unit that holds the two polyenes parallel to each other, only allowing + 2cycloadditions to occur. A common spacer used in these reactions is the .2aracyclophane system. This is sufficiently rigid and can hold the polyene tails in close enough proximity for the cycloadditions to occur. MacGillivray and colleagues have demonstrated that a supramolecular approach to covalent synthesis in the organized, solvent-free environment of the solid state can provide a solution to the problem of organizing two polyenes for an intramolecular reaction to give a ladderane. Specifically, by taking an approach to control reactivity in solids by using molecules that serve as linear templates, they have demonstrated the utility of cocrystallization of resorcinol (1,3-benzenediol), or a derivative, with an all-''trans''-bis(4-pyridyl)poly-''m''-ene (4-pyr-poly-''m''-ene) produces a four-component molecular assembly, 2(resorcinol)·2(4-pyr-poly-''m''-ene), in which each resorcinol preorganizes, through two O—H···N hydrogen-bonding interactions, two poly-''m''-enes for +2photoaddition. The two polyenes are positioned by the templates such that the C=C bonds of the olefins lie parallel and separated by < 4.2 Å, a position suitable for the photoreaction. UV irradiation of the solid produces the targeted 'n''adderane, with the C=C bonds reacting to form the fused cyclobutane framework. Broadband UV-irradiation of two such hydrogen-bonded, four-component supramolecular assemblies furnishes the corresponding ladderanes stereospecifically and in quantitative yield in gram quantities.
MacGillivray and colleagues have demonstrated that a supramolecular approach to covalent synthesis in the organized, solvent-free environment of the solid state can provide a solution to the problem of organizing two polyenes for an intramolecular reaction to give a ladderane. Specifically, by taking an approach to control reactivity in solids by using molecules that serve as linear templates, they have demonstrated the utility of cocrystallization of resorcinol (1,3-benzenediol), or a derivative, with an all-''trans''-bis(4-pyridyl)poly-''m''-ene (4-pyr-poly-''m''-ene) produces a four-component molecular assembly, 2(resorcinol)·2(4-pyr-poly-''m''-ene), in which each resorcinol preorganizes, through two O—H···N hydrogen-bonding interactions, two poly-''m''-enes for +2photoaddition. The two polyenes are positioned by the templates such that the C=C bonds of the olefins lie parallel and separated by < 4.2 Å, a position suitable for the photoreaction. UV irradiation of the solid produces the targeted 'n''adderane, with the C=C bonds reacting to form the fused cyclobutane framework. Broadband UV-irradiation of two such hydrogen-bonded, four-component supramolecular assemblies furnishes the corresponding ladderanes stereospecifically and in quantitative yield in gram quantities.
Biological background
 Ladderanes were first identified in a rare group of anaerobic ammonium oxidizing ( anammox) bacteria belonging to the phylum Planctomycetota. These bacteria sequester the catabolic anammox reactions to intracellular compartments called anammoxosomes. The anammox process involves the oxidation of
Ladderanes were first identified in a rare group of anaerobic ammonium oxidizing ( anammox) bacteria belonging to the phylum Planctomycetota. These bacteria sequester the catabolic anammox reactions to intracellular compartments called anammoxosomes. The anammox process involves the oxidation of ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
to nitrogen gas with nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
as the final electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mista ...
. Intermediates in this process are two highly toxic compounds, hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
(N2H4) and hydroxylamine (NH2OH). The oxidation process involves the generation of a proton gradient on the intracytoplasmic face of the anammoxosome. Dissipation of the proton gradient is coupled to the phosphorylation of ADP
Adp or ADP may refer to:
Aviation
* Aéroports de Paris, airport authority for the Parisian region in France
* Aeropuertos del Perú, airport operator for airports in northern Peru
* SLAF Anuradhapura, an airport in Sri Lanka
* Ampara Air ...
through membrane-bound ATPase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, SV40 T-antigen, ATP hydrolase, complex V (mitochondrial electron transport), (Ca2+ + Mg2+)-ATPase, HCO3−-ATPase, adenosine triphosphatase) are ...
s.
Anammoxosomes are enriched in the ladderane lipids shown at right. Analysis of the anammoxosome membranes from the bacterial species '' Brocadia anammoxidans'' and '' Kuenenia stuttgartiensis'' has revealed that ladderanes constitute more than 50% of membrane lipids. The high abundance of ladderane lipids in the anammoxosome results in an exceptionally dense membrane with reduced permeability. The reduced permeability may decrease the passive diffusion of protons across the membrane that would dissipate the electrochemical gradient. This would be especially detrimental to anammox bacteria, due to the relatively slow anammox metabolism. The decreased permeability has also been hypothesized to sequester the highly toxic and mutagenic intermediates, hydrazine and hydroxylamine, which can readily diffuse through biomembranes. The loss of these key intermediates would damage key cellular components such as DNA, as well as reduce the catabolic efficiency of the cell.
Synthesis of ladderane lipids
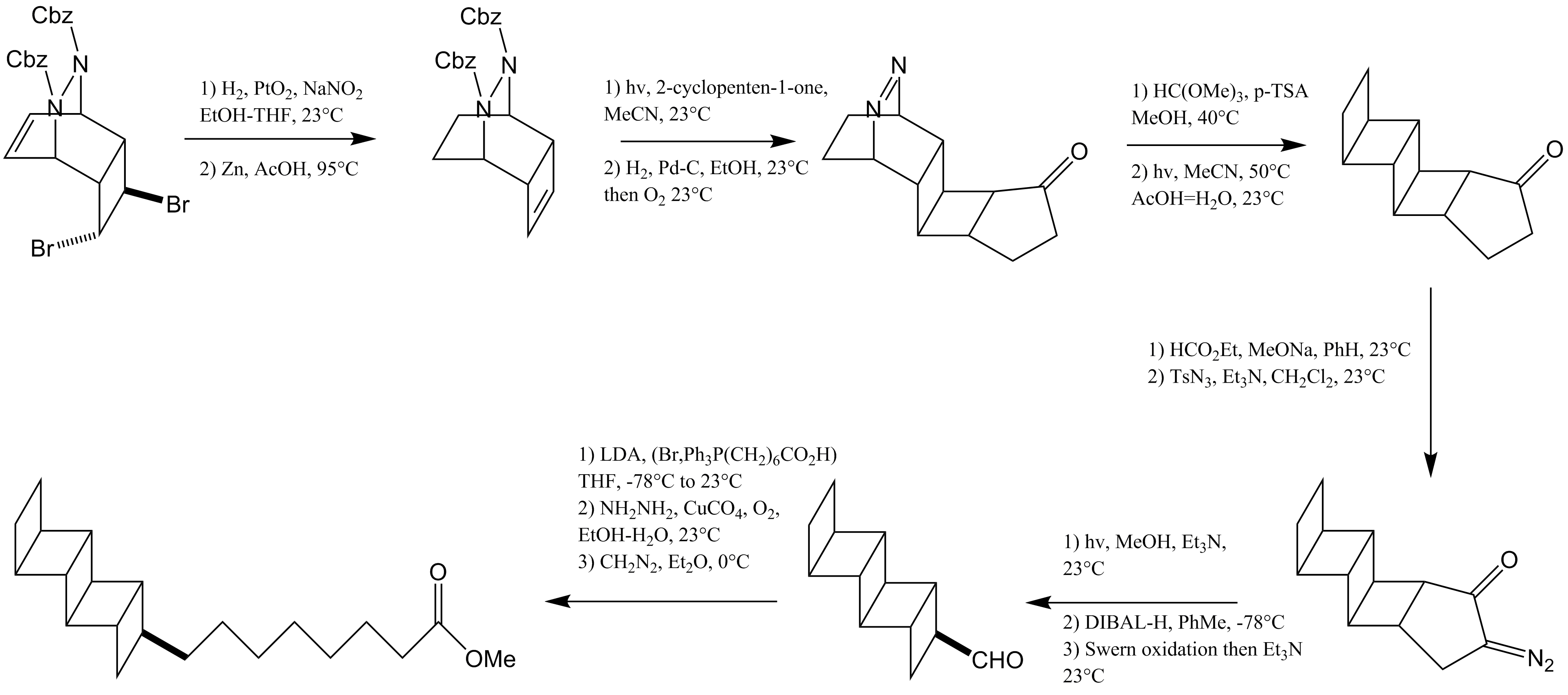
A naturally occurring ladderane lipid, named pentacycloanammoxic acid, has been synthesized by Corey and coworkers. The first step in this reaction involves bromination followed by cyclization of cyclooctatetraene to form a cyclohexadiene. This cyclohexadiene is trapped by dibenzyl azodicarboxylate. Functional group modifications are made to produce a cyclobutane which is elaborated through a +2photocycloaddition with a cyclopentenone to produce a second cyclobutane ring. Protection of the carbonyl group, followed by a N2 extrusion reaction, yields two more fused cyclobutane rings. The final cyclobutane is formed by a Wolff rearrangement, and the alkyl chain is installed by a Wittig olefination. In 2016, Burns and co-workers at Stanford University reported an enantioselective synthesis of both the and ladderane lipid tails and their incorporation into a full phosphatidylcholine lipid.
Both routes leverage a small ladderene building block bicyclo .2.0exene prepared by a Ramberg–Bäcklund reaction. The route to a ladderane-containing fatty acid involves dimerization of this intermediate to form an all-''anti'' ladderane hydrocarbon. C–H chlorination by a manganese porphyrin catalyst and subsequent elimination introduces unsaturation to produce a ladderene. Hydroboration and a Zweifel reaction install the linear alkyl group.
In 2016, Burns and co-workers at Stanford University reported an enantioselective synthesis of both the and ladderane lipid tails and their incorporation into a full phosphatidylcholine lipid.
Both routes leverage a small ladderene building block bicyclo .2.0exene prepared by a Ramberg–Bäcklund reaction. The route to a ladderane-containing fatty acid involves dimerization of this intermediate to form an all-''anti'' ladderane hydrocarbon. C–H chlorination by a manganese porphyrin catalyst and subsequent elimination introduces unsaturation to produce a ladderene. Hydroboration and a Zweifel reaction install the linear alkyl group.
hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
-mediated deoxygenation reaction followed by hydrogenation with Crabtree's catalyst effects reduction to the cyclohexane ring.
References
{{Reflist Cyclobutanes Polycyclic nonaromatic hydrocarbons