Krische Allylation on:
[Wikipedia]
[Google]
[Amazon]
The Krische allylation involves the 
 The excellent functional group compatibility of the Krische allylation combined with the tractability of the
The excellent functional group compatibility of the Krische allylation combined with the tractability of the 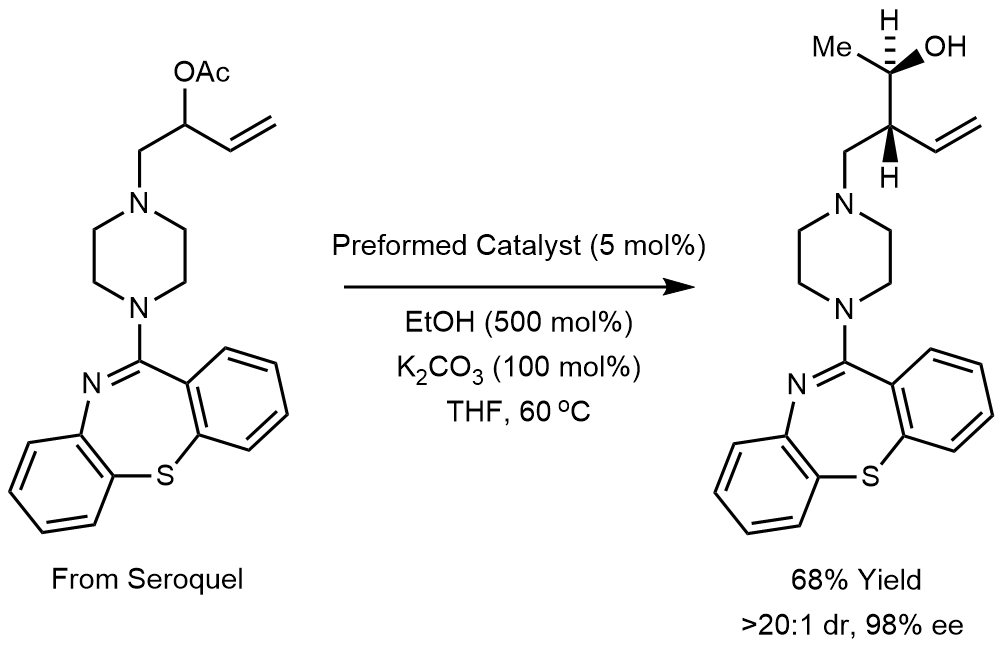 The figure below shows some of the different allyl donors that have been used in the Krische allylation. These methods are summarized in the review literature.
The figure below shows some of the different allyl donors that have been used in the Krische allylation. These methods are summarized in the review literature.

 The mechanism of the Krische allylation has been corroborated by DFT calculations. Entry into the
The mechanism of the Krische allylation has been corroborated by DFT calculations. Entry into the 
 The Krische bisallylation has been applied to the synthesis of
The Krische bisallylation has been applied to the synthesis of 
Krische Group Website
Iridium Catalysis Organometallic chemistry Organic reactions
enantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
iridium-catalyzed addition of an allyl group to an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
or an alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols (precluding alcohol to aldehyde oxidation).

Background
Enantioselective carbonyl allylations are frequently applied to the synthesis ofpolyketide
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynth ...
natural products. In 1978, Hoffmann reported the first asymmetric carbonyl allylation using a chiral allylmetal reagent, an allylborane derived from camphor. Subsequently, other chiral allylmetal reagents were developed by Kumada, Roush, Brown, Leighton, and others. These methods utilize preformed allyl metal reagents and generate stoichiometric quantities of metal byproducts.
In 1991, Yamamoto disclosed the first catalytic enantioselective method for carbonyl allylation In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reage ...
, which employed a chiral boron Lewis acid-catalyst in combination with allyltrimethylsilane. Numerous catalytic enantioselective methods for carbonyl allylation followed, including work by Umani-Ronchi and Keck. While these methods had a significant impact, they do not circumvent the use of preformed allylmetal reagents. Catalytic variants of the Nozaki-Hiyama-Kishi reaction represent an alternative method for asymmetric carbonyl allylation, but stoichiometric metallic reductants are required.
Whereas the allylmetal reagents used in these first-generation technologies are often difficult to prepare and handle, the Krische allylation exploits highly tractable allylic acetates. Additionally, the Krische allylation avoids the use of preformed allyl metal reagents or metallic reductants and chiral auxiliaries, significantly reducing waste generation.
Reaction features
The Krische allylation involves “ transfer hydrogenative” carbon-carbon bond formations. In a series of papers published in the early 2000s, Krische and coworkers demonstrated that allenes,dienes Dienes may refer to:
* Dienes (surname), including a list of people with the name
* the plural of diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon at ...
, and allyl acetates could be converted to transient allylmetal nucleophiles via hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
, transfer hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and redu ...
or hydrogen auto-transfer. This strategy for enantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
carbonyl allylation avoids preformed organometallic reagents or metallic reductants. A remarkable feature of these reactions is the ability to conduct carbonyl allylation from the alcohol oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
. Due to a kinetic preference for primary alcohol dehydrogenation, diols containing both primary and secondary alcohols undergo site-selective carbonyl allylation at the primary alcohol without the need for protecting groups
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
. Additionally, by using alcohol reactants, the use of chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
α-stereogenic aldehydes, which are prone to racemization In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred too as a racemic mixture (i.e. conta ...
, can be avoided.
 The excellent functional group compatibility of the Krische allylation combined with the tractability of the
The excellent functional group compatibility of the Krische allylation combined with the tractability of the allyl acetate
Allyl acetate is an organic compound with formula C3H5OC(O)CH3. This colourless liquid is a precursor to especially allyl alcohol, which is a useful industrial intermediate. It is the acetate ester of allyl alcohol.
Preparation
Allyl acetate i ...
pronucleophiles enables the use of allyl donors bearing highly complex nitrogen-rich substituents.
 The figure below shows some of the different allyl donors that have been used in the Krische allylation. These methods are summarized in the review literature.
The figure below shows some of the different allyl donors that have been used in the Krische allylation. These methods are summarized in the review literature.

Mechanism
The active catalyst in the Krische allylation is a cyclometallated π-allyliridium ''C,O''-benzoate complex. This complex can be generated in situ or can be isolated via precipitation or conventional chromatography on silica gel. The mechanism of the Krische allylation has been corroborated by DFT calculations. Entry into the
The mechanism of the Krische allylation has been corroborated by DFT calculations. Entry into the catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
involves protonation of the cyclometallated π-allyliridium precatalyst to generate the iridium alkoxide I. β-Hydride elimination of alkoxide I generates the aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
, which dissociates to form the iridium hydride III. Deprotonation of the iridium hydride III provides an anionic iridium(I) species IV, which upon oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxid ...
to the allyl donor forms the π-allyliridium complex V. Association of the aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
to the σ-allyliridium species VI triggers carbonyl addition by way of the six-centered transition structure VII to form the homoallylic alkoxide VIII. The homoallylic alkoxide VIII is stable with respect to beta-hydride elimination
β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the β-carbon. For instance butyl groups can undergo th ...
due to coordination of the double bond with the metal. Exchange with the primary alcohol reactant regenerates the iridium alkoxide I and releases the reaction product.

Applications in synthesis
Iridium-catalyzed transfer-hydrogenative carbonyl allylation method has been applied to the synthesis of polyketide natural products. Some examples are shown below. In every case, the target compound was prepared in significantly fewer steps than was previously achieved. For example, total syntheses of roxaticin, bryostatin and cryptocaryol were accomplished via double Krische allylation of 1,3-propane diol. This method was also used in the synthesis of mandelalide A. The Krische bisallylation has been applied to the synthesis of
The Krische bisallylation has been applied to the synthesis of psymberin
Psymberin, also known as irciniastatin A, is a cytotoxin derived from sea sponges. It was discovered by two independent research groups, one led by Dr. Phil Crews and one led by Dr. Jean Schmidt, in 2004. Psymberin was found to be highly bioacti ...
in 17 LLS and 32 total steps. Through the use of the Krische allylation, this synthesis was accomplished via a much shorter route than previous syntheses. The Krische allylation to his synthesis of callyspongiolide using the chiral SEGPHOS
SEGPHOS is a chiral ligand developed by Takasago that is used in asymmetric synthesis. It was developed after BINAP
BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This chiral diphosphine ligand ...
catalyst complex. ] In 2018, Harran also prepared callyspongiolide using the Krische allylation as a convergent method for fragment union. Double crotylation was used by Krische to prepare 6-deoxyerythronolide B and swinholide A.

Related articles
*Organostannane addition Organostannane addition reactions comprise the nucleophilic addition of an allyl-, allenyl-, or propargylstannane to an aldehyde, imine, or, in rare cases, a ketone. The reaction is widely used for carbonyl allylation.
Organostannane addition to ...
* Carbonyl allylation In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reage ...
References
{{Reflist, 30emExternal links
Krische Group Website
Iridium Catalysis Organometallic chemistry Organic reactions