|
Β-hydride Elimination
╬▓-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the ╬▓-carbon. For instance butyl groups can undergo this reaction but methyl groups cannot. The metal complex must have an empty (or vacant) site ''cis'' to the alkyl group for this reaction to occur. Moreover, for facile cleavage of the CŌĆōH bond, a d electron pair is needed for donation into the Žā* orbital of the CŌĆōH bond. Thus, d0 metals alkyls are generally more stable to ╬▓-hydride elimination than d2 and higher metal alkyls and may form isolable agostic complexes, even if an empty coordination site is available. The ╬▓-hydride elimination can either be a vital step in a reaction or an unproductive side reaction. The Shell higher olefin process relies on ╬▓-hydride elimination to produce ╬▒-olefins which are used to produce detergents. Illustrative of a sometimes undesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a Ring (chemistry), ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to other molecular fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenium Trichloride
Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3┬Ę''x''H2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry. Preparation and properties Anhydrous ruthenium(III) chloride is usually prepared by heating powdered ruthenium metal with chlorine. In the original synthesis, the chlorination was conducted in the presence of carbon monoxide, the product being carried by the gas stream and crystallising upon cooling. Two allotropes of RuCl3 are known. The black ╬▒-form adopts the CrCl3-type structure with long Ru-Ru contacts of 346 pm. This allotrope has honeycomb layers of Ru3+ which are surrounded with an octahedral cage of ClŌłÆ anions. The ruthenium cations are magnetic residing in a low-spin J~1/2 ground st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coplanar
In geometry, a set of points in space are coplanar if there exists a geometric plane that contains them all. For example, three points are always coplanar, and if the points are distinct and non-collinear, the plane they determine is unique. However, a set of four or more distinct points will, in general, not lie in a single plane. Two lines in three-dimensional space are coplanar if there is a plane that includes them both. This occurs if the lines are parallel, or if they intersect each other. Two lines that are not coplanar are called skew lines. Distance geometry provides a solution technique for the problem of determining whether a set of points is coplanar, knowing only the distances between them. Properties in three dimensions In three-dimensional space, two linearly independent vectors with the same initial point determine a plane through that point. Their cross product is a normal vector to that plane, and any vector orthogonal to this cross product through the initia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications. A trimethylsilyl group bonded to a methyl group forms tetramethylsilane, which is abbreviated as TMS as well. Compounds with trimethylsilyl groups are not normally found in nature. Chemists sometimes use a trimethylsilylating reagent to derivatize rather non-volatile compounds such as certain alcohols, phenols, or carboxylic acids by substituting a trimethylsilyl group for a hydrogen in the hydroxyl groups on the compounds. This way trimethylsiloxy groups minus;O-Si(CH3)3are formed on the molecule. A couple of examples of trimethylsilylating agents include trimethylsilyl chloride and bis(trimethylsilyl)acetamide. Trime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''-b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira (ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCO databases, ProQuest databases, Index Medicus/MEDLINE/PubMed, and the Science Citation Index Expanded. According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 16.383. Editors-in-chief The following people are or have been editor-in-chief: * 1879ŌĆō1880 ŌĆō Hermann Endemann * 1880ŌĆō1881 ŌĆō Gideon E. Moore * 1881ŌĆō1882 ŌĆō Hermann Endemann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
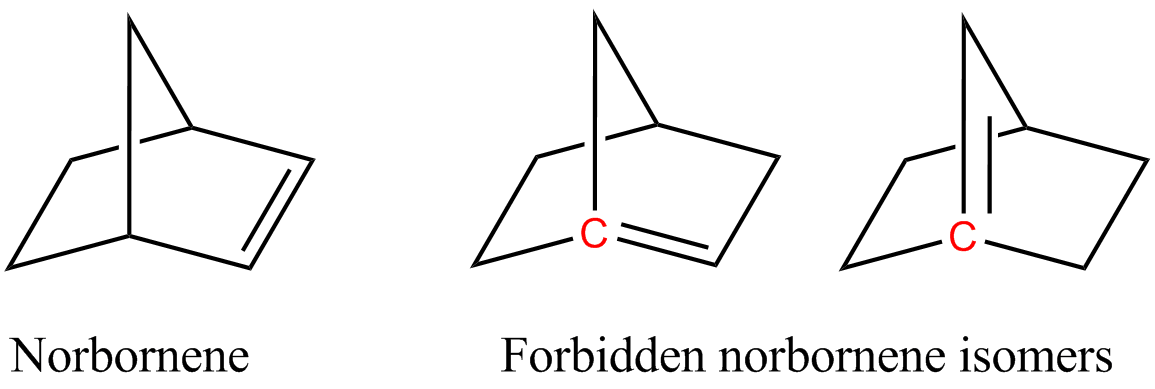
Bredt's Rule
Bredt's rule is an empirical observation in organic chemistry that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. It primarily relates to bridgeheads with carbon-carbon and carbon-nitrogen double bonds. For example, two of the following isomers of norbornene violate Bredt's rule, which makes them too unstable to prepare: In the figure, the bridgehead atoms involved in Bredt's rule violation are highlighted in red. Bredt's rule is a consequence of the fact that having a double bond on a bridgehead, carbons from which three bonds radiate and which the rings share a single covalent bond, would be equivalent to having a trans double bond on a ring, which is not stable for small rings (fewer than eight atoms) due to a combination of ring strain, and angle strain (nonplanar alkene). The p orbitals of the bridgehead ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neopentyl
Pentyl is a five-carbon alkyl group or substituent with chemical formula -C5H11. It is the substituent form of the alkane pentane. In older literature, the common non-systematic name amyl was often used for the pentyl group. Conversely, the name pentyl was used for several five-carbon branched alkyl groups, distinguished by various prefixes. The nomenclature has now reversed, with "amyl" being more often used to refer to the terminally branched group also called isopentyl, as in amobarbital. A cyclopentyl group is a ring with the formula -C5H9. The name is also used for the pentyl radical, a pentyl group as an isolated molecule. This free radical is only observed in extreme conditions. Its formula is often written "ŌĆó" or "ŌĆóŌĆåŌüĀ" to indicate that it has one unsatisfied valence bond. Older "pentyl" groups The following names are still used sometimes: Pentyl radical The free radical pentyl was studied by J. Pacansky and A. Gutierrez in 1983. The radical was obtained by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |