|
Organostannane Addition
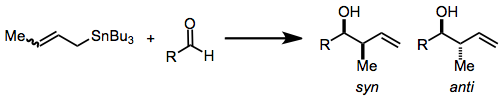
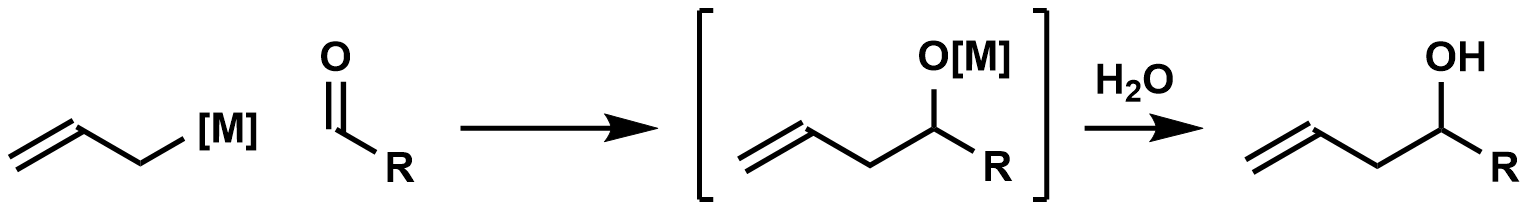
Organostannane addition reactions comprise the nucleophilic addition of an allyl-, allenyl-, or propargylstannane to an aldehyde, imine, or, in rare cases, a ketone. The reaction is widely used for carbonyl allylation. Organostannane addition to carbonyl groups constitutes one of the most common and efficient methods for the construction of contiguous, oxygen-containing stereocenters in organic molecules. As many molecules containing this motif—polypropionates and polyacetates, for instance—are desired by natural products chemists, the title reaction has become important synthetically and has been heavily studied over the years. Substituted allylstannanes may create one or two new stereocenters, often with a very high degree of stereocontrol. Organostannanes are known for their stability, ease of handling, and selective reactivity. Chiral allylstannanes often react with good stereoselectivity to give single diastereomers. Models explaining the sense of selectivity are relia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Allylation
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent. Enantioselective versions In 1978, Hoffmann reported the first asymmetric carbonyl allylation using a chiral allylmetal reagent, an allylborane derived from camphor. Such methods utilize preformed allyl metal reagents. The approach is well developed using allyl boranesDenmark, S. E.; Almstead, N. G. In ''Modern Carbonyl Chemistry''; Otera, J., Ed.; Wiley-VCH: Weinheim, 2000; Chapter 10. As illustrated by the Keck allylation, catalytic enantioselective additions of achiral allylmetal reagents to carbonyl compounds also are possible by organostannane additions. Allylic boronate and -borane reagents have also been developed for enantioselective addition to carbonyls—in this class of reactions, the allylic boron reagent confers ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krische Allylation
The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols (precluding alcohol to aldehyde oxidation). Background Enantioselective carbonyl allylations are frequently applied to the synthesis of polyketide natural products. In 1978, Hoffmann reported the first asymmetric carbonyl allylation using a chiral allylmetal reagent, an allylborane derived from camphor. Subsequently, other chiral allylmetal reagents were developed by Kumada, Roush, Brown, Leighton, and others. These methods utilize preformed allyl meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrolein
Acrolein (systematic name: propenal) is the simplest unsaturated aldehyde. It is a colorless liquid with a piercing, acrid smell. The smell of burnt fat (as when cooking oil is heated to its smoke point) is caused by glycerol in the burning fat breaking down into acrolein. It is produced industrially from propylene and mainly used as a biocide and a building block to other chemical compounds, such as the amino acid methionine. History Acrolein was first named and characterized as an aldehyde by the Swedish chemist Jöns Jacob Berzelius in 1839. He had been working with it as a thermal degradation product of glycerol, a material used in the manufacture of soap. The name is a contraction of ‘acrid’ (referring to its pungent smell) and ‘oleum’ (referring to its oil-like consistency). In the 20th century, acrolein became an important intermediate for the industrial production of acrylic acid and acrylic plastics. Production Acrolein is prepared industrially by oxidation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Bromide
Magnesium bromide is a chemical compound of magnesium and bromine, with the chemical formula . It is white and deliquescent crystalline solid. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. It is water-soluble and somewhat soluble in alcohol. It can be found naturally in small amounts in some minerals such as: bischofite and carnallite, and in sea water, such as that of the Dead Sea.Gruyter, W. Concise Encyclopedia Chemistry, Walter de Gruyter & Company: Berlin, 1993; 612Lewis, R.J. Hawley’s Condensed Chemical Dictionary, 15th ed.; John Wiley &Sons Inc.:New York, 2007; 777 Synthesis Magnesium bromide can be synthesized by treating with magnesium oxide (and related basic salts) with hydrobromic acid. It can also be made by reacting magnesium carbonate and hydrobromic acids, and collecting the solid left after evaporation. As suggested by its easy conversion to various hydrates, anhydrous is a Lewis acid. In the coordination p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |