Ketocarotenoid on:
[Wikipedia]
[Google]
[Amazon]
 Carotenoids (), also called tetraterpenoids, are yellow, orange, and red organic
Carotenoids (), also called tetraterpenoids, are yellow, orange, and red organic 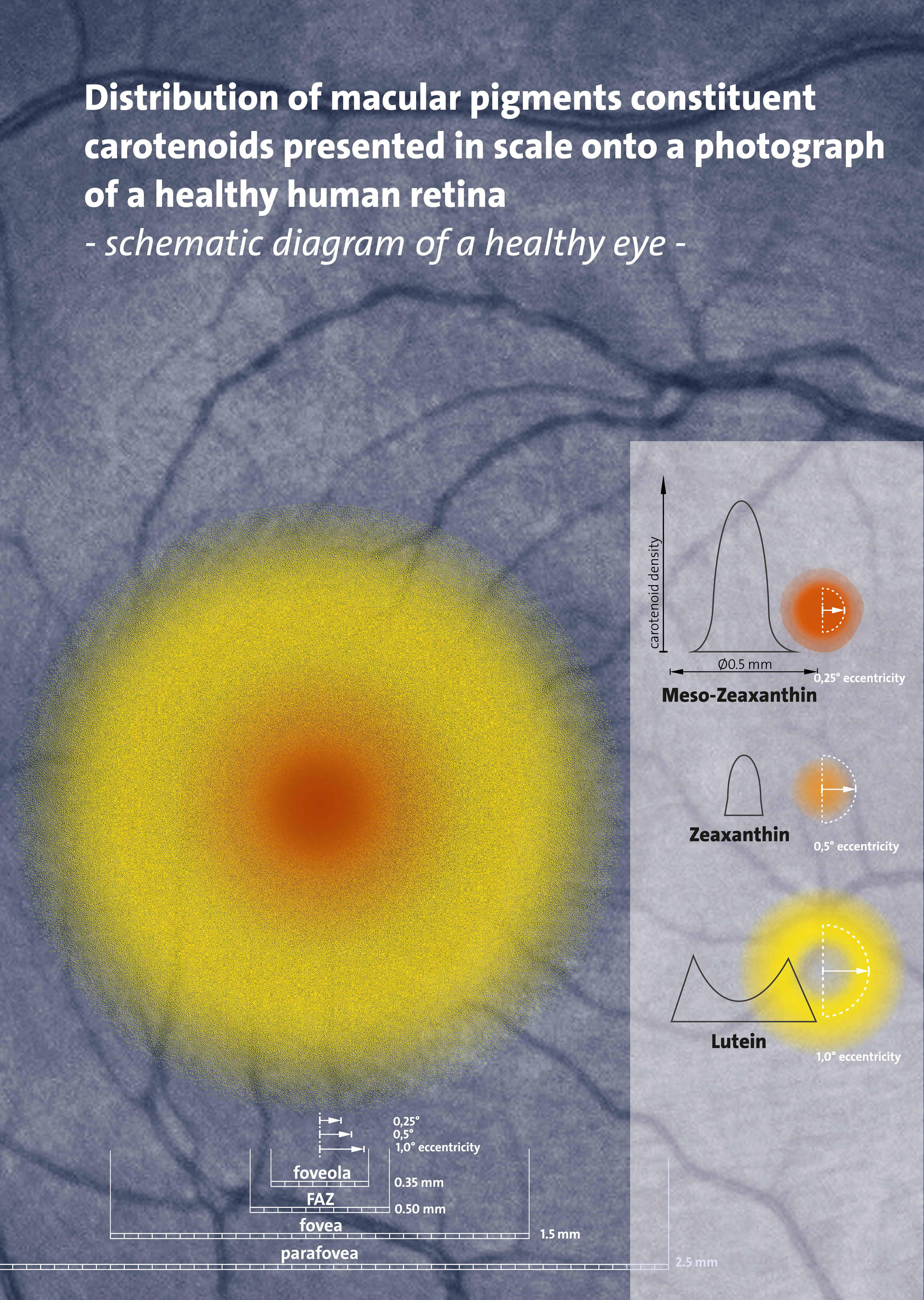 Carotenoids serve two key roles in plants and algae: they absorb light energy for use in
Carotenoids serve two key roles in plants and algae: they absorb light energy for use in
 Carotenoids (), also called tetraterpenoids, are yellow, orange, and red organic
Carotenoids (), also called tetraterpenoids, are yellow, orange, and red organic pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
s that are produced by plant
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclu ...
s and algae, as well as several bacteria, and fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from ...
. Carotenoids give the characteristic color to pumpkins, carrots, parsnip
The parsnip ('' Pastinaca sativa'') is a root vegetable closely related to carrot and parsley, all belonging to the flowering plant family Apiaceae. It is a biennial plant usually grown as an annual. Its long taproot has cream-colored skin an ...
s, corn, tomato
The tomato is the edible berry of the plant ''Solanum lycopersicum'', commonly known as the tomato plant. The species originated in western South America, Mexico, and Central America. The Mexican Nahuatl word gave rise to the Spanish word ...
es, canaries, flamingo
Flamingos or flamingoes are a type of wading bird in the family Phoenicopteridae, which is the only extant family in the order Phoenicopteriformes. There are four flamingo species distributed throughout the Americas (including the Caribbea ...
s, salmon
Salmon () is the common name for several commercially important species of euryhaline ray-finned fish from the family Salmonidae, which are native to tributaries of the North Atlantic (genus ''Salmo'') and North Pacific (genus '' Oncorhy ...
, lobster, shrimp, and daffodil
''Narcissus'' is a genus of predominantly spring flowering perennial plants of the amaryllis family, Amaryllidaceae. Various common names including daffodil,The word "daffodil" is also applied to related genera such as ''Sternbergia'', ''Ism ...
s. Carotenoids can be produced from fats and other basic organic metabolic building blocks by all these organisms. It is also produced by endosymbiotic
An ''endosymbiont'' or ''endobiont'' is any organism that lives within the body or cells of another organism most often, though not always, in a mutualistic relationship.
(The term endosymbiosis is from the Greek: ἔνδον ''endon'' "within ...
bacteria in whiteflies
Whiteflies are Hemipterans that typically feed on the undersides of plant leaves. They comprise the family Aleyrodidae, the only family in the superfamily Aleyrodoidea. More than 1550 species have been described.
Description and taxonomy
The ...
. Carotenoids from the diet are stored in the fatty tissues of animals, and exclusively carnivorous animals obtain the compounds from animal fat. In the human diet, absorption of carotenoids is improved when consumed with fat in a meal. Cooking carotenoid-containing vegetables in oil and shredding the vegetable both increase carotenoid bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. Ho ...
.
There are over 1,100 known carotenoids which can be further categorized into two classes, xanthophyll
Xanthophylls (originally phylloxanthins) are yellow pigments that occur widely in nature and form one of two major divisions of the carotenoid group; the other division is formed by the carotenes. The name is from Greek (, "yellow") and (, "lea ...
s (which contain oxygen) and carotenes (which are purely hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s and contain no oxygen). All are derivatives of tetraterpene
Tetraterpenes are terpenes consisting of eight isoprene units and have the molecular formula C40H64. Tetraterpenoids (including many carotenoids) are tetraterpenes that have been chemically modified, as indicated by the presence of oxygen-contain ...
s, meaning that they are produced from 8 isoprene molecules and contain 40 carbon atoms. In general, carotenoids absorb wavelengths ranging from 400 to 550 nanometers (violet to green light). This causes the compounds to be deeply colored yellow, orange, or red. Carotenoids are the dominant pigment in autumn leaf coloration of about 15-30% of tree species, but many plant colors, especially reds and purples, are due to polyphenol
Polyphenols () are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some o ...
s.
 Carotenoids serve two key roles in plants and algae: they absorb light energy for use in
Carotenoids serve two key roles in plants and algae: they absorb light energy for use in photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
, and they provide photoprotection
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative st ...
via non-photochemical quenching
Non-photochemical quenching (NPQ) is a mechanism employed by plants and algae to protect themselves from the adverse effects of high light intensity. It involves the quenching of singlet excited state chlorophylls (Chl) via enhanced internal con ...
. Carotenoids that contain unsubstituted beta-ionone rings (including β-carotene
β-Carotene is an organic, strongly coloured red-orange pigment abundant in fungi, plants, and fruits. It is a member of the carotenes, which are terpenoids (isoprenoids), synthesized biochemically from eight isoprene units and thus having 40 ...
, α-carotene, β-cryptoxanthin, and γ-carotene
γ-Carotene is a carotenoid, and is a biosynthetic intermediate for cyclized carotenoid synthesis in plants. It is formed from cyclization of lycopene by lycopene cyclase epsilon.Rodriguez-Concepcion M, Stange C. Biosynthesis of carotenoids in ...
) have vitamin A
Vitamin A is a fat-soluble vitamin and an essential nutrient for humans. It is a group of organic compounds that includes retinol, retinal (also known as retinaldehyde), retinoic acid, and several provitamin A carotenoids (most notably ...
activity (meaning that they can be converted to retinol
Retinol, also called vitamin A1, is a fat-soluble vitamin in the vitamin A family found in food and used as a dietary supplement. As a supplement it is used to treat and prevent vitamin A deficiency, especially that which results in xeroph ...
). In the eye, lutein
Lutein (;"Lutein"
''meso''-zeaxanthin, and
 The basic building blocks of carotenoids are isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). These two isoprene isomers are used to create various compounds depending on the biological pathway used to synthesize the isomers. Plants are known to use two different pathways for IPP production: the cytosolic mevalonic acid pathway (MVA) and the plastidic methylerythritol 4-phosphate (MEP). In animals, the production of
The basic building blocks of carotenoids are isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). These two isoprene isomers are used to create various compounds depending on the biological pathway used to synthesize the isomers. Plants are known to use two different pathways for IPP production: the cytosolic mevalonic acid pathway (MVA) and the plastidic methylerythritol 4-phosphate (MEP). In animals, the production of
 The structure of carotenoids allows for biological abilities, including
The structure of carotenoids allows for biological abilities, including

 Carotenoids belong to the category of tetraterpenoids (i.e., they contain 40 carbon atoms, being built from four
Carotenoids belong to the category of tetraterpenoids (i.e., they contain 40 carbon atoms, being built from four
zeaxanthin
Zeaxanthin is one of the most common carotenoids in nature, and is used in the xanthophyll cycle. Synthesized in plants and some micro-organisms, it is the pigment that gives paprika (made from bell peppers), corn, saffron, goji ( wolfberries ...
are present as macular pigments whose importance in visual function, as of 2016, remains under clinical research
Clinical research is a branch of healthcare science that determines the safety and effectiveness ( efficacy) of medications, devices, diagnostic products and treatment regimens intended for human use. These may be used for prevention, treatm ...
.
Biosynthesis
cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell mem ...
starts by creating IPP and DMAPP using the MVA. For carotenoid production plants use MEP to generate IPP and DMAPP. The MEP pathway results in a 5:1 mixture of IPP:DMAPP. IPP and DMAPP undergo several reactions, resulting in the major carotenoid precursor, geranylgeranyl diphosphate (GGPP). GGPP can be converted into carotenes or xanthophylls by undergoing a number of different steps within the carotenoid biosynthetic pathway.
MEP pathway
Glyceraldehyde 3-phosphate
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GA3P, GADP, GAP, TP, GALP or PGAL, is a metabolite that occurs as an intermediate in several central pathways of all organisms.Nelson, D ...
and pyruvate, intermediates of photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
, are converted to deoxy-D-xylulose 5-phosphate (DXP) using the catalyst DXP synthase
In enzymology, a 1-deoxy--xylulose-5-phosphate synthase () is an enzyme in the non-mevalonate pathway that catalyzes the chemical reaction
:pyruvate + -glyceraldehyde 3-phosphate \rightleftharpoons 1-deoxy--xylulose 5-phosphate + CO2
Thus, t ...
(DXS). DXP reductoisomerase
DXP reductoisomerase (1-deoxy-D-xylulose 5-phosphate reductoisomerase or DXR) is an enzyme that interconverts 1-deoxy-D-xylulose 5-phosphate (DXP) and 2-C-methyl-D-erythritol 4-phosphate (MEP).
Image:DOXP.png, 1-Deoxy-D-xylulose 5-phosphate
...
reduces and rearranges the molecules within DXP in the presence of NADPH, forming MEP. Next, MEP is converted to 4-(cytidine 5’-diphospho)-2-C-methyl-D-erythritol (CDP-ME) in the presence of CTP via the enzyme MEP cytidylyltransferase. CDP-ME is then converted, in the presence of ATP, to 2-phospho-4-(cytidine 5’-diphospho)-2-C-methyl-D-erythritol (CDP-ME2P). The conversion to CDP-ME2P is catalyzed by the enzyme CDP-ME kinase. Next, CDP-ME2P is converted to 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MECDP). This reaction occurs when MECDP synthase catalyzes the reaction and CMP is eliminated from the CDP-ME2P molecule. MECDP is then converted to (e)-4-hydroxy-3-methylbut-2-en-1-yl diphosphate (HMBDP) via HMBDP synthase in the presence of flavodoxin Flavodoxins (Fld) are small, soluble electron-transfer proteins. Flavodoxins contains flavin mononucleotide as prosthetic group. The structure of flavodoxin is characterized by a five-stranded parallel beta sheet, surrounded by five alpha helices. T ...
and NADPH. HMBDP is reduced to IPP in the presence of ferredoxin and NADPH by the enzyme HMBDP reductase. The last two steps involving HMBPD synthase and reductase can only occur in completely anaerobic
Anaerobic means "living, active, occurring, or existing in the absence of free oxygen", as opposed to aerobic which means "living, active, or occurring only in the presence of oxygen." Anaerobic may also refer to:
* Anaerobic adhesive, a bonding a ...
environments. IPP is then able to isomerize to DMAPP via IPP isomerase.
Carotenoid biosynthetic pathway
Two GGPP molecules condense viaphytoene synthase
Phytoene synthase (, ''prephytoene-diphosphate synthase'', ''15-cis-phytoene synthase'', ''PSase'', ''geranylgeranyl-diphosphate geranylgeranyltransferase'') is a transferase enzyme involved in the biosynthesis of carotenoids. It catalyzes the conv ...
(PSY), forming the 15-cis isomer of phytoene
Phytoene () is a 40-carbon intermediate in the biosynthesis of carotenoids. The synthesis of phytoene is the first committed step in the synthesis of carotenoids in plants. Phytoene is produced from two molecules of geranylgeranyl pyrophosphate (G ...
. PSY belongs to the squalene/phytoene synthase family
The squalene/phytoene synthase family represents proteins that catalyze the head-to-head condensation of C15 and C20 prenyl units (i.e. farnesyl diphosphate and genranylgeranyl diphosphate). This enzymatic step constitutes part of steroid and c ...
and is homologous to squalene synthase
Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step react ...
that takes part in steroid biosynthesis. The subsequent conversion of phytoene into all-trans-lycopene
Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene (from the neo-Latin '' Lycopersicum'', the tomato species) is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables.
Occu ...
depends on the organism. Bacteria and fungi employ a single enzyme, the bacterial phytoene desaturase (CRTI) for the catalysis. Plants and cyanobacteria however utilize four enzymes for this process. The first of these enzymes is a plant-type phytoene desaturase which introduces two additional double bonds into 15-cis-phytoene by dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At ...
and isomerizes two of its existing double bonds from trans to cis producing 9,15,9’-tri-cis-ζ-carotene. The central double bond of this tri-cis-ζ-carotene is isomerized by the zeta-carotene isomerase Z-ISO and the resulting 9,9'-di-cis-ζ-carotene is dehydrogenated again via a ζ-carotene desaturase (ZDS). This again introduces two double bonds, resulting in 7,9,7’,9’-tetra-cis-lycopene. CRTISO
Prolycopene isomerase (, ''CRTISO'', ''carotene cis-trans isomerase'', ''ZEBRA2 (gene)'', ''carotene isomerase'', ''carotenoid isomerase'') is an enzyme with systematic name ''7,9,7',9'-tetracis-lycopene cis-trans-isomerase''. This enzyme catalyse ...
, a carotenoid isomerase, is needed to convert the cis
Cis or cis- may refer to:
Places
* Cis, Trentino, in Italy
* In Poland:
** Cis, Świętokrzyskie Voivodeship, south-central
** Cis, Warmian-Masurian Voivodeship, north
Math, science and biology
* cis (mathematics) (cis(''θ'')), a trigonome ...
-lycopene into an all-trans lycopene in the presence of reduced FAD
A fad or trend is any form of collective behavior that develops within a culture, a generation or social group in which a group of people enthusiastically follow an impulse for a short period.
Fads are objects or behaviors that achieve short- ...
.
This all-trans lycopene is cyclized; cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
gives rise to carotenoid diversity, which can be distinguished based on the end groups. There can be either a beta ring or an epsilon ring, each generated by a different enzyme ( lycopene beta-cyclase eta-LCYor lycopene epsilon-cyclase psilon-LCY. α-Carotene is produced when the all-trans lycopene first undergoes reaction with epsilon-LCY then a second reaction with beta-LCY; whereas β-carotene
β-Carotene is an organic, strongly coloured red-orange pigment abundant in fungi, plants, and fruits. It is a member of the carotenes, which are terpenoids (isoprenoids), synthesized biochemically from eight isoprene units and thus having 40 ...
is produced by two reactions with beta-LCY. α- and β-Carotene are the most common carotenoids in the plant photosystem
Photosystems are functional and structural units of protein complexes involved in photosynthesis. Together they carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons. Photosystems ...
s but they can still be further converted into xanthophylls by using beta-hydrolase and epsilon-hydrolase, leading to a variety of xanthophylls.
Regulation
It is believed that both DXS and DXR are rate-determining enzymes, allowing them to regulate carotenoid levels. This was discovered in an experiment where DXS and DXR were genetically overexpressed, leading to increased carotenoid expression in the resulting seedlings. Also, J-protein (J20) and heat shock protein 70 (Hsp70) chaperones are thought to be involved in post-transcriptional regulation of DXS activity, such that mutants with defective J20 activity exhibit reduced DXS enzyme activity while accumulating inactive DXS protein. Regulation may also be caused by externaltoxin
A toxin is a naturally occurring organic poison produced by metabolic activities of living cells or organisms. Toxins occur especially as a protein or conjugated protein. The term toxin was first used by organic chemist Ludwig Brieger (1849 ...
s that affect enzymes and proteins required for synthesis. Ketoclomazone is derived from herbicides applied to soil and binds to DXP synthase. This inhibits DXP synthase, preventing synthesis of DXP and halting the MEP pathway. The use of this toxin leads to lower levels of carotenoids in plants grown in the contaminated soil. Fosmidomycin
Fosmidomycin is an antibiotic that was originally isolated from culture broths of bacteria of the genus '' Streptomyces''. It specifically inhibits DXP reductoisomerase, a key enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. It ...
, an antibiotic, is a competitive inhibitor
Competitive inhibition is interruption of a chemical pathway owing to one chemical substance inhibiting the effect of another by competing with it for binding or bonding. Any metabolic or chemical messenger system can potentially be affected b ...
of DXP reductoisomerase due to its similar structure to the enzyme. Application of said antibiotic prevents reduction of DXP, again halting the MEP pathway.
Structure and function
 The structure of carotenoids allows for biological abilities, including
The structure of carotenoids allows for biological abilities, including photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
, photoprotection
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative st ...
, plant coloration, and cell signaling
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellula ...
.
The general structure of the carotenoid is a polyene
In organic chemistry, polyenes are poly- unsaturated, organic compounds that contain at least three alternating double () and single () carbon–carbon bonds. These carbon–carbon double bonds interact in a process known as conjugation, result ...
chain consisting of 9-11 double bonds and possibly terminating in rings. This structure of conjugated double bonds
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as ...
leads to a high reducing potential, or the ability to transfer electrons throughout the molecule. Carotenoids can transfer excitation energy in one of two ways: 1) singlet-singlet energy transfer from carotenoid to chlorophyll, and 2) triplet-triplet energy transfer from chlorophyll to carotenoid. The singlet-singlet energy transfer is a lower energy state transfer and is used during photosynthesis. The length of the polyene tail enables light absorbance in the photosynthetic range; once it absorbs energy it becomes excited, then transfers the excited electrons to the chlorophyll for photosynthesis. The triplet-triplet transfer is a higher energy state and is essential in photoprotection. Light produces damaging species during photosynthesis, with the most damaging being reactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
(ROS). As these high energy ROS are produced in the chlorophyll the energy is transferred to the carotenoid’s polyene tail and undergoes a series of reactions in which electrons are moved between the carotenoid bonds in order to find the most balanced (lowest energy) state for the carotenoid.
The length of carotenoids also has a role in plant coloration, as the length of the polyene tail determines which wavelengths of light the plant will absorb. Wavelengths that are not absorbed are reflected and are what we see as the color of a plant. Therefore, differing species will contain carotenoids with differing tail lengths allowing them to absorb and reflect different colors.
Carotenoids also participate in different types of cell signaling. They are able to signal the production of absicisic acid, which regulates plant growth, seed dormancy
Seed dormancy is an evolutionary adaptation that prevents seeds from germinating during unsuitable ecological conditions that would typically lead to a low probability of seedling survival. Dormant seeds do not germinate in a specified period of ...
, embryo maturation and germination, cell division
Cell division is the process by which a parent cell divides into two daughter cells. Cell division usually occurs as part of a larger cell cycle in which the cell grows and replicates its chromosome(s) before dividing. In eukaryotes, there ar ...
and elongation, floral growth, and stress responses.
Properties

 Carotenoids belong to the category of tetraterpenoids (i.e., they contain 40 carbon atoms, being built from four
Carotenoids belong to the category of tetraterpenoids (i.e., they contain 40 carbon atoms, being built from four terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ...
units each containing 10 carbon atoms). Structurally, carotenoids take the form of a polyene
In organic chemistry, polyenes are poly- unsaturated, organic compounds that contain at least three alternating double () and single () carbon–carbon bonds. These carbon–carbon double bonds interact in a process known as conjugation, result ...
hydrocarbon chain which is sometimes terminated by rings, and may or may not have additional oxygen atoms attached.
* Carotenoids with molecules containing oxygen, such as lutein
Lutein (;"Lutein"

 The most common carotenoids include lycopene and the vitamin A precursor β-carotene. In plants, the xanthophyll
The most common carotenoids include lycopene and the vitamin A precursor β-carotene. In plants, the xanthophyll
zeaxanthin
Zeaxanthin is one of the most common carotenoids in nature, and is used in the xanthophyll cycle. Synthesized in plants and some micro-organisms, it is the pigment that gives paprika (made from bell peppers), corn, saffron, goji ( wolfberries ...
, are known as xanthophyll
Xanthophylls (originally phylloxanthins) are yellow pigments that occur widely in nature and form one of two major divisions of the carotenoid group; the other division is formed by the carotenes. The name is from Greek (, "yellow") and (, "lea ...
s.
* The unoxygenated (oxygen free) carotenoids such as α-carotene, β-carotene
β-Carotene is an organic, strongly coloured red-orange pigment abundant in fungi, plants, and fruits. It is a member of the carotenes, which are terpenoids (isoprenoids), synthesized biochemically from eight isoprene units and thus having 40 ...
, and lycopene
Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene (from the neo-Latin '' Lycopersicum'', the tomato species) is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables.
Occu ...
, are known as carotenes. Carotenes typically contain only carbon and hydrogen (i.e., are hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s), and are in the subclass of unsaturated hydrocarbon
300px, Structure of an ethene molecule, the simplest unsaturated hydrocarbon
Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. The term "unsaturated" means more hydrogen atoms may ...
s.
Their color, ranging from pale yellow through bright orange to deep red, is directly linked to their structure. Xanthophylls are often yellow, hence their class name. The double carbon-carbon bonds interact with each other in a process called conjugation
Conjugation or conjugate may refer to:
Linguistics
* Grammatical conjugation, the modification of a verb from its basic form
* Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
* Complex conjugation, the chang ...
, which allows electrons in the molecule to move freely across these areas of the molecule. As the number of conjugated double bonds increases, electrons associated with conjugated systems have more room to move, and require less energy to change states. This causes the range of energies of light absorbed by the molecule to decrease. As more wavelengths of light are absorbed from the longer end of the visible spectrum, the compounds acquire an increasingly red appearance.
Carotenoids are usually lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipo ...
due to the presence of long unsaturated aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, ...
chains as in some fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
s. The physiological absorption of these fat-soluble vitamin
A vitamin is an organic molecule (or a set of molecules closely related chemically, i.e. vitamers) that is an essential micronutrient that an organism needs in small quantities for the proper functioning of its metabolism. Essential nutri ...
s in humans and other organisms depends directly on the presence of fats and bile salt
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.
P ...
s.
Foods
Beta-carotene, found in pumpkins, sweet potato, carrots andwinter squash
Winter squash is an annual fruit representing several squash species within the genus ''Cucurbita''. Late-growing, less symmetrical, odd-shaped, rough or warty varieties, small to medium in size, but with long-keeping qualities and hard rinds, are ...
, is responsible for their orange-yellow colors. Dried carrots have the highest amount of carotene of any food per 100-gram serving, measured in retinol activity equivalents (provitamin A equivalents). Vietnamese gac
GAC or Gac may refer to:
Companies and organisations
* GAC Group, a Chinese automotive company based in Guangzhou, Guangdong
* GAC Ireland, an Irish bus manufacturer established with Bombardier (1980–1986)
* Games Administration Committee, ...
fruit contains the highest known concentration of the carotenoid lycopene
Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene (from the neo-Latin '' Lycopersicum'', the tomato species) is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables.
Occu ...
. Although green, kale, spinach, collard greens
Collard is a group of certain loose-leafed cultivars of ''Brassica oleracea'', the same species as many common vegetables including cabbage ( Capitata group) and broccoli ( Italica group). Collard is a member of the Viridis group of ''Brassica ...
, and turnip greens
The turnip or white turnip (''Brassica rapa'' subsp. ''rapa'') is a root vegetable commonly grown in temperate climates worldwide for its white, fleshy taproot. The word ''turnip'' is a compound of ''turn'' as in turned/rounded on a lathe and ...
contain substantial amounts of beta-carotene. The diet of flamingo
Flamingos or flamingoes are a type of wading bird in the family Phoenicopteridae, which is the only extant family in the order Phoenicopteriformes. There are four flamingo species distributed throughout the Americas (including the Caribbea ...
s is rich in carotenoids, imparting the orange-colored feathers of these birds.
Morphology
Carotenoids are located primarily outside the cell nucleus in different cytoplasm organelles, lipid droplets, Microbody, cytosomes and granules. They have been visualised and quantified by raman spectroscopy in an algal cell. With the development of Monoclonal antibody, monoclonal antibodies to ''trans-''lycopene
Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene (from the neo-Latin '' Lycopersicum'', the tomato species) is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables.
Occu ...
it was possible to localise this carotenoid in different animal and human cells.
Oxygenation
Carotenoids play an important role in biological oxygenation. In plant cells they are involved in the control of trans-membrane transport of molecular oxygen released inphotosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
.
In animals carotenoids play an important role to support oxygen in its transport, storage and metabolism.
Transport
Carotenoids are hydrophobic and are typically present in plasma lipoproteins and cellular lipid structures. Since molecular oxygen is also a Hydrophobe, hydrophobic molecule, lipids provide a more favorable environment for O2 solubility than in aqueous mediums. By protecting lipids from free-radical damage, which generate charged Lipid peroxidation, lipid peroxides and other oxidised derivatives, carotenoids support crystalline architecture and hydrophobicity of lipoproteins and cellular lipid structures, hence oxygen solubility and its diffusion therein.Storage
It was first suggested that carotenoids can be involved in the intracellular depot of oxygen in 1973 by V.N. Karnaukhov. Later it was discovered that carotenoids can also stimulate the formation of intracellular lipid droplets, which can store additional molecular oxygen. These properties of carotenoids help animals to adapt to environmental stresses, high altitude, intracellular infections and other Hypoxia (environmental), hypoxicRespiration
Carotenoids, by increasing oxygen diffusion and the oxygen carrying capacity of plasma lipoproteins, can stimulate oxygen delivery into body tissues. This improves tissue and cellular oxygenation and stimulates the growth and respiration of Mitochondrion, mitochondria.Synergetic modality
Oxygen is required in many intracellular reactions including hydroxylation, which is important for metabolic activation of prodrugs and Hormone, prohormones, such as vitamin D3. Carotenoids not only provide support for intracellular oxygenation but can also improve efficacy of these molecules. Carotenoids can form physical complexes with different molecules. With hydrophobic molecules this could be self-assembly. With Amphiphile, amphiphilic or Hydrophile, hydrophilic compounds the use of lycosome or Supercritical carbon dioxide, supercritical CO2 technologies, or other methods, are required.] Carotenoids in these complexes provide a new Therapy, modality of supporting and boosting tissue oxygenation, which could be synergistically beneficial to the therapeutic objectives of different nutraceutical or Medication, pharmaceutical molecules.Physiological effects
Reviews of epidemiology, epidemiological studies seeking correlations between carotenoid consumption in food and clinical outcomes have come to various conclusions: * A 2015 review found that foods high in carotenoids appear to be protective against head and neck cancers. * Another 2015 review looking at whether carotenoids can prevent prostate cancer found that while several studies found correlations between diets rich in carotenoids appeared to have a protective effect, evidence is lacking to determine whether this is due to carotenoids per se. * A 2014 review found no correlation between consumption of foods high in carotenoids and vitamin A and the risk of getting Parkinson's disease. * Another 2014 review found no conflicting results in studies of dietary consumption of carotenoids and the risk of getting breast cancer. Carotenoids are also important components of the dark brown pigment melanin, which is found in hair, skin, and eyes. Melanin absorbs high-energy light and protects these organs from intracellular damage. * Several studies have observed positive effects of high-carotenoid diets on the texture, clarity, color, strength, and elasticity of skin. * A 1994 study noted that high carotenoid diets helped reduce symptoms of eyestrain (dry eye, headaches, and blurred vision) and improve night vision. Humans and other animals are mostly incapable of synthesizing carotenoids, and must obtain them through their diet. Carotenoids are a common and often ornamental feature in animals. For example, the pink color ofsalmon
Salmon () is the common name for several commercially important species of euryhaline ray-finned fish from the family Salmonidae, which are native to tributaries of the North Atlantic (genus ''Salmo'') and North Pacific (genus '' Oncorhy ...
, and the red coloring of cooked lobsters and scales of the yellow morph of common wall lizards are due to carotenoids. It has been proposed that carotenoids are used in ornamental traits (for extreme examples see puffin birds) because, given their physiological and chemical properties, they can be used as visible indicators of individual health, and hence are used by animals when selecting potential mates.
Plant colors

 The most common carotenoids include lycopene and the vitamin A precursor β-carotene. In plants, the xanthophyll
The most common carotenoids include lycopene and the vitamin A precursor β-carotene. In plants, the xanthophyll lutein
Lutein (;"Lutein"
chlorophyll. When chlorophyll is not present, as in autumn foliage, the yellows and oranges of the carotenoids are predominant. For the same reason, carotenoid colors often predominate in ripe fruit after being unmasked by the disappearance of chlorophyll.
Carotenoids are responsible for the brilliant yellows and oranges that tint deciduous foliage (such as dying Autumn leaf color, autumn leaves) of certain hardwood species as hickory, hickories, ash (tree), ash, maple, yellow poplar, aspen, birch, black cherry, sycamore, Populus sect. Aegiros, cottonwood, sassafras, and alder. Carotenoids are the dominant pigment in autumn leaf coloration of about 15-30% of tree species. However, the reds, the purples, and their blended combinations that decorate autumn foliage usually come from another group of pigments in the cells called anthocyanins. Unlike the carotenoids, these pigments are not present in the leaf throughout the growing season, but are actively produced towards the end of summer.
Carotenoid Terpenoids
Carotenoid gene in aphids
International Carotenoid Society
* {{Authority control Bioindicators Carotenoids, Photosynthesis
Bird colors and sexual selection
Dietary carotenoids and their metabolic derivatives are responsible for bright yellow to red coloration in birds. Studies estimate that around 2956 modern bird species display carotenoid coloration and that the ability to italicize these pigments for external coloration has evolved independently many times thought avian evolutionary history. Carotenoid coloration exhibits high levels of sexual dimorphism, meaning that male birds tend to display more vibrant coloration than females of the same species. These differences arise due to the selection of yellow and red coloration in males by Mate choice, female preference. In many species of birds, females invest greater time and resources into raising offspring than their male partners. Therefore, it is imperative that female birds carefully select high quality mates. Current literature supports the theory that vibrant carotenoid coloration is correlated with male quality—either though direct effects on immune function and oxidative stress, or through a connection between carotenoid metabolizing pathways and pathways for cellular respiration.Sexual signaling
It is generally considered that sexually selected traits, such as carotenoid-based coloration, evolve because they are honest signals of phenotypic and genetic quality. For instance, among males of the bird species ''great tit, Parus major'', the more colorfully ornamented males produce sperm that is better protected against oxidative stress due to increased presence of carotenoid antioxidants. However, there is also evidence that attractive male coloration may be a faulty signal of male quality. Among stickleback fish, males that are more attractive to females due to carotenoid colorants appear to under-allocate carotenoids to their germline cells.Kim SY, Velando A. Attractive male sticklebacks carry more oxidative DNA damage in the soma and germline. J Evol Biol. 2020 Jan;33(1):121-126. doi: 10.1111/jeb.13552. Epub 2019 Nov 7. PMID: 31610052 Since carotinoids are beneficial antioxidants, their under-allocation to germline cells can lead to increased oxidative DNA damage (naturally occurring), DNA damage to these cells. Therefore, female sticklebacks may risk fertility and the viability of their offspring by choosing redder, but more deteriorated partners with reduced sperm quality.Aroma chemicals
Products of carotenoid degradation such as ionones, damascones and damascenones are also important fragrance chemicals that are used extensively in the perfumes and fragrance industry. Both β-damascenone and β-ionone although low in concentration in rose distillates are the key odor-contributing compounds in flowers. In fact, the sweet floral smells present in black tea, aged tobacco, grape, and many fruits are due to the aromatic compounds resulting from carotenoid breakdown.Disease
Some carotenoids are produced by bacteria to protect themselves from oxidative immune attack. The ''aureus'' (golden) pigment that gives some strains of ''Staphylococcus aureus'' their name is a carotenoid called staphyloxanthin. This carotenoid is a virulence factor with an antioxidant action that helps the microbe evade death byreactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
used by the host immune system.
Naturally occurring carotenoids
* Hydrocarbons ** Lycopersene 7,8,11,12,15,7',8',11',12',15'-Decahydro-γ,γ-carotene ** Phytofluene ** Lycopene ** Hexahydrolycopene 15-''cis''-7,8,11,12,7',8'-Hexahydro-γ,γ-carotene ** Torulene 3',4'-Didehydro-β,γ-carotene ** α-Zeacarotene 7',8'-Dihydro-ε,γ-carotene ** α-Carotene ** β-Carotene ** γ-Carotene ** δ-Carotene ** ε-Carotene ** ζ-Carotene * Alcohols ** Alloxanthin ** Bacterioruberin 2,2'-Bis(3-hydroxy-3-methylbutyl)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-γ,γ-carotene-1,1'-diol ** Cynthiaxanthin ** Pectenoxanthin ** Cryptomonaxanthin (3R,3'R)-7,8,7',8'-Tetradehydro-β,β-carotene-3,3'-diol ** Crustaxanthin β,-Carotene-3,4,3',4'-tetrol ** Gazaniaxanthin (3R)-5'-cis-β,γ-Caroten-3-ol ** OH-Chlorobactene 1',2'-Dihydro-f,γ-caroten-1'-ol ** Loroxanthin β,ε-Carotene-3,19,3'-triol ** Lutein (3R,3′R,6′R)-β,ε-carotene-3,3′-diol ** Lycoxanthin γ,γ-Caroten-16-ol ** Rhodopin 1,2-Dihydro-γ,γ-caroten-l-ol ** Rhodopinol a.k.a. Warmingol 13-''cis''-1,2-Dihydro-γ,γ-carotene-1,20-diol ** Saproxanthin 3',4'-Didehydro-1',2'-dihydro-β,γ-carotene-3,1'-diol ** Zeaxanthin * Glycosides ** Oscillaxanthin 2,2'-Bis(β-L-rhamnopyranosyloxy)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-γ,γ-carotene-1,1'-diol ** Phleixanthophyll 1'-(β-D-Glucopyranosyloxy)-3',4'-didehydro-1',2'-dihydro-β,γ-caroten-2'-ol * Ethers ** Rhodovibrin 1'-Methoxy-3',4'-didehydro-1,2,1',2'-tetrahydro-γ,γ-caroten-1-ol ** Spheroidene 1-Methoxy-3,4-didehydro-1,2,7',8'-tetrahydro-γ,γ-carotene * Epoxides ** Diadinoxanthin 5,6-Epoxy-7',8'-didehydro-5,6-dihydro—carotene-3,3-diol ** Luteoxanthin 5,6: 5',8'-Diepoxy-5,6,5',8'-tetrahydro-β,β-carotene-3,3'-diol ** Mutatoxanthin ** Citroxanthin ** Zeaxanthin furanoxide 5,8-Epoxy-5,8-dihydro-β,β-carotene-3,3'-diol ** Neochrome (Carotenoid), Neochrome 5',8'-Epoxy-6,7-didehydro-5,6,5',8'-tetrahydro-β,β-carotene-3,5,3'-triol ** Foliachrome ** Trollichrome ** Vaucheriaxanthin 5',6'-Epoxy-6,7-didehydro-5,6,5',6'-tetrahydro-β,β-carotene-3,5,19,3'-tetrol * Aldehydes ** Rhodopinal ** Warmingone 13-cis-1-Hydroxy-1,2-dihydro-γ,γ-caroten-20-al ** Torularhodinaldehyde 3',4'-Didehydro-β,γ-caroten-16'-al * Acids and acid esters ** Torularhodin 3',4'-Didehydro-β,γ-caroten-16'-oic acid ** Torularhodin methyl ester Methyl 3',4'-didehydro-β,γ-caroten-16'-oate * Ketones ** Astacene ** Astaxanthin ** Canthaxanthin a.k.a. Aphanicin, Chlorellaxanthin β,β-Carotene-4,4'-dione ** Capsanthin (3R,3'S,5'R)-3,3'-Dihydroxy-β,κ-caroten-6'-one ** Capsorubin (3S,5R,3'S,5'R)-3,3'-Dihydroxy-κ,κ-carotene-6,6'-dione ** Cryptocapsin (3'R,5'R)-3'-Hydroxy-β,κ-caroten-6'-one ** 2,2'-Diketospirilloxanthin 1,1'-Dimethoxy-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-γ,γ-carotene-2,2'-dione ** Echinenone β,β-Caroten-4-one ** 3'-Hydroxyechinenone ** Flexixanthin 3,1'-Dihydroxy-3',4'-didehydro-1',2'-dihydro-β,γ-caroten-4-one ** 3-OH-Canthaxanthin a.k.a. Adonirubin a.k.a. Phoenicoxanthin 3-Hydroxy-β,β-carotene-4,4'-dione ** Hydroxyspheriodenone 1'-Hydroxy-1-methoxy-3,4-didehydro-1,2,1',2',7',8'-hexahydro-γ,γ-caroten-2-one ** Okenone 1'-Methoxy-1',2'-dihydro-c,γ-caroten-4'-one ** Pectenolone 3,3'-Dihydroxy-7',8'-didehydro-β,β-caroten-4-one ** Phoeniconone a.k.a. Dehydroadonirubin 3-Hydroxy-2,3-didehydro-β,β-carotene-4,4'-dione ** Phoenicopterone β,ε-caroten-4-one ** Rubixanthone 3-Hydroxy-β,γ-caroten-4'-one ** Siphonaxanthin 3,19,3'-Trihydroxy-7,8-dihydro-β,ε-caroten-8-one * Esters of alcohols ** Astacein 3,3'-Bispalmitoyloxy-2,3,2',3'-tetradehydro-β,β-carotene-4,4'-dione or 3,3'-dihydroxy-2,3,2',3'-tetradehydro-β,β-carotene-4,4'-dione dipalmitate ** Fucoxanthin 3'-Acetoxy-5,6-epoxy-3,5'-dihydroxy-6',7'-didehydro-5,6,7,8,5',6'-hexahydro-β,β-caroten-8-one ** Isofucoxanthin 3'-Acetoxy-3,5,5'-trihydroxy-6',7'-didehydro-5,8,5',6'-tetrahydro-β,β-caroten-8-one ** Physalien ** Siphonein 3,3'-Dihydroxy-19-lauroyloxy-7,8-dihydro-β,ε-caroten-8-one or 3,19,3'-trihydroxy-7,8-dihydro-β,ε-caroten-8-one 19-laurate * Apocarotenoids ** β-Apo-2'-carotenal 3',4'-Didehydro-2'-apo-b-caroten-2'-al ** Apo-2-lycopenal ** Apo-6'-lycopenal 6'-Apo-y-caroten-6'-al ** Azafrinaldehyde 5,6-Dihydroxy-5,6-dihydro-10'-apo-β-caroten-10'-al ** Bixin 6'-Methyl hydrogen 9'-cis-6,6'-diapocarotene-6,6'-dioate ** Citranaxanthin 5',6'-Dihydro-5'-apo-β-caroten-6'-one or 5',6'-dihydro-5'-apo-18'-nor-β-caroten-6'-one or 6'-methyl-6'-apo-β-caroten-6'-one ** Crocetin 8,8'-Diapo-8,8'-carotenedioic acid ** Crocetinsemialdehyde 8'-Oxo-8,8'-diapo-8-carotenoic acid ** Crocin Digentiobiosyl 8,8'-diapo-8,8'-carotenedioate ** Hopkinsiaxanthin 3-Hydroxy-7,8-didehydro-7',8'-dihydro-7'-apo-b-carotene-4,8'-dione or 3-hydroxy-8'-methyl-7,8-didehydro-8'-apo-b-carotene-4,8'-dione ** Methyl apo-6'-lycopenoate Methyl 6'-apo-y-caroten-6'-oate ** Paracentrone 3,5-Dihydroxy-6,7-didehydro-5,6,7',8'-tetrahydro-7'-apo-b-caroten-8'-one or 3,5-dihydroxy-8'-methyl-6,7-didehydro-5,6-dihydro-8'-apo-b-caroten-8'-one ** Sintaxanthin 7',8'-Dihydro-7'-apo-b-caroten-8'-one or 8'-methyl-8'-apo-b-caroten-8'-one * Nor- and seco-carotenoids ** Actinioerythrin 3,3'-Bisacyloxy-2,2'-dinor-b,b-carotene-4,4'-dione ** β-Carotenone 5,6:5',6'-Diseco-b,b-carotene-5,6,5',6'-tetrone ** Peridinin 3'-Acetoxy-5,6-epoxy-3,5'-dihydroxy-6',7'-didehydro-5,6,5',6'-tetrahydro-12',13',20'-trinor-b,b-caroten-19,11-olide ** Pyrrhoxanthininol 5,6-epoxy-3,3'-dihydroxy-7',8'-didehydro-5,6-dihydro-12',13',20'-trinor-b,b-caroten-19,11-olide ** Semi-α-carotenone 5,6-Seco-b,e-carotene-5,6-dione ** Semi-β-carotenone 5,6-seco-b,b-carotene-5,6-dione or 5',6'-seco-b,b-carotene-5',6'-dione ** Triphasiaxanthin 3-Hydroxysemi-b-carotenone 3'-Hydroxy-5,6-seco-b,b-carotene-5,6-dione or 3-hydroxy-5',6'-seco-b,b-carotene-5',6'-dione * Retro-carotenoids and retro-apo-carotenoids ** Eschscholtzxanthin 4',5'-Didehydro-4,5'-retro-b,b-carotene-3,3'-diol ** Eschscholtzxanthone 3'-Hydroxy-4',5'-didehydro-4,5'-retro-b,b-caroten-3-one ** Rhodoxanthin 4',5'-Didehydro-4,5'-retro-b,b-carotene-3,3'-dione ** Tangeraxanthin 3-Hydroxy-5'-methyl-4,5'-retro-5'-apo-b-caroten-5'-one or 3-hydroxy-4,5'-retro-5'-apo-b-caroten-5'-one * Higher carotenoids ** Nonaprenoxanthin 2-(4-Hydroxy-3-methyl-2-butenyl)-7',8',11',12'-tetrahydro-e,y-carotene ** Decaprenoxanthin 2,2'-Bis(4-hydroxy-3-methyl-2-butenyl)-e,e-carotene ** C.p. 450 2-[4-Hydroxy-3-(hydroxymethyl)-2-butenyl]-2'-(3-methyl-2-butenyl)-b,b-carotene ** C.p. 473 2'-(4-Hydroxy-3-methyl-2-butenyl)-2-(3-methyl-2-butenyl)-3',4'-didehydro-l',2'-dihydro-β,γ-caroten-1'-ol ** Bacterioruberin 2,2'-Bis(3-hydroxy-3-methylbutyl)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-γ,γ-carotene-1,1'-diolSee also
* List of phytochemicals in food * CRT (genetics), gene cluster responsible for the biosynthesis of carotenoids * E number#E100–E199 (colours) * PhytochemistryReferences
External links
Carotenoid Terpenoids
Carotenoid gene in aphids
International Carotenoid Society
* {{Authority control Bioindicators Carotenoids, Photosynthesis