|
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> \o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
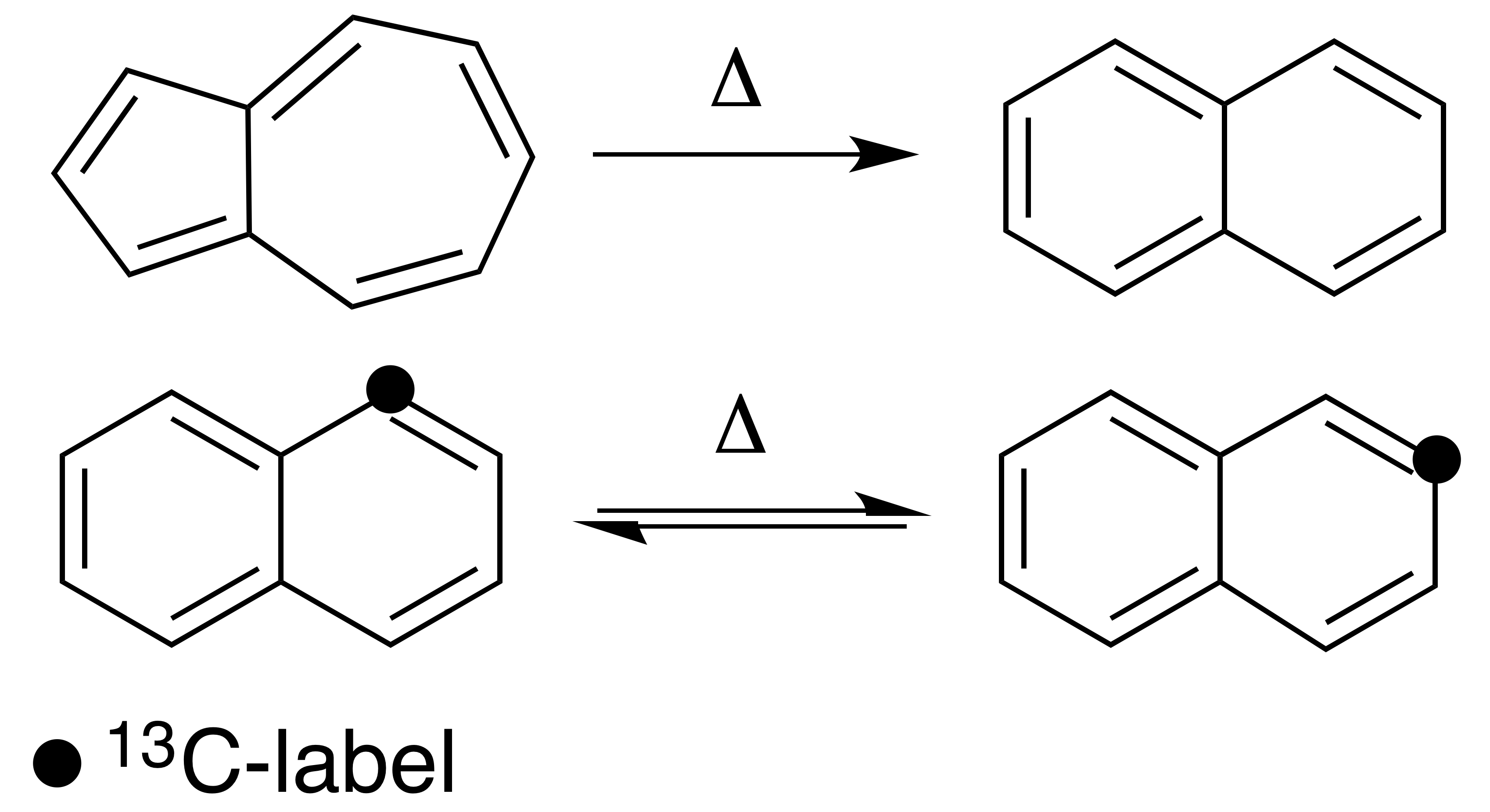
Thermal Rearrangement Of Aromatic Hydrocarbons
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs ( automerization). The general reaction schemes of the two types are illustrated in Figure 1. This class of reactions was uncovered through studies on the automerization of naphthalene as well as the isomerization of unsubstituted azulene, to naphthalene. Research on thermal rearrangements of aromatic hydrocarbons has since been expanded to isomerizations and automerizations of benzene and polycyclic aromatic hydrocarbons. Mechanisms Automerizations The first proposed mechanism for a thermal rearrangement of an aromatic compound was for the automerization of naphth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back-for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resveratrol
Resveratrol (3,5,4′-trihydroxy-''trans''-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacteria or fungi. Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts. Although commonly used as a dietary supplement and studied in laboratory models of human diseases, there is no high-quality evidence that resveratrol improves lifespan or has a substantial effect on any human disease. Research Resveratrol has been studied for its potential therapeutic use, with little evidence of anti-disease effects or health benefits in humans. Cardiovascular disease There is no evidence of benefit from resveratrol in people who already have heart disease. A 2018 meta-analysis found no effect on systolic or diastolic blood pressure; a sub-analysis revealed a 2 mmHg decrease in systolic pressure only from res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rearrangement Reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule, hence these reactions are usually intramolecular. In the example below, the substituent R moves from carbon atom 1 to carbon atom 2: :\underset\ce\ce\underset\ce\ce Intermolecular rearrangements also take place. A rearrangement is not well represented by simple and discrete electron transfers (represented by curved arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner-Meerwein rearrangement, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
I-butane
Isobutane, also known as ''i''-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colourless, odourless gas. It is the simplest alkane with a tertiary carbon atom. Isobutane is used as a precursor molecule in the petrochemical industry, for example in the synthesis of isooctane. Production Isobutane is obtained by isomerization of butane. : Uses Isobutane is the principal feedstock in alkylation units of refineries. Using isobutane, gasoline-grade "blendstocks" are generated with high branching for good combustion characteristics. Typical products created with isobutane are 2,4-dimethylpentane and especially 2,2,4-trimethylpentane. Solvent In the Chevron Phillips slurry process for making high-density polyethylene, isobutane is used as a diluent. As the slurried polyethylene is removed, isobutane is "flashed" off, and condensed, and recycled back into the loop reactor for this purpose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shell Higher Olefin Process
The Shell higher olefin process (SHOP) is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Royal Dutch Shell.''Industrial Organic Chemistry'', Klaus Weissermel, Hans-Jurgen Arpe John Wiley & Sons; 3rd 1997 The olefin products are converted to fatty aldehydes and then to fatty alcohols, which are precursors plasticizers and detergents. The annual global production of olefines through this method is over one million tonnes.Keim, W. (2013), ''Oligomerization of Ethylene to α-Olefins: Discovery and Development of the Shell Higher Olefin Process (SHOP)''. Angew. Chem. Int. Ed., 52: 12492–12496. History The process was discovered by chemists at Shell Development Emeryville in 1968. At the time ecological considerations demanded the replacement of branched fatty alcohols used widely in detergents, by linear fatty alcohols because the biodegradation of the branched compounds was slow, causing foa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octane Rating
An octane rating, or octane number, is a standard measure of a fuel's ability to withstand compression in an internal combustion engine without detonating. The higher the octane number, the more compression the fuel can withstand before detonating. Octane rating does not relate directly to the power output or the energy content of the fuel per unit mass or volume, but simply indicates gasoline's capability against compression. Whether or not a higher octane fuel improves or impairs an engine's performance depends on the design of the engine. In broad terms, fuels with a higher octane rating are used in higher-compression gasoline engines, which may yield higher power for these engines. Such higher power comes from the fuel's higher compression by the engine design, and not directly from the gasoline. In contrast, fuels with lower octane (but higher cetane numbers) are ideal for diesel engines because diesel engines (also called compression-ignition engines) do not compress the fue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saccharide Chemistry
Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the detection, synthesis, structure, and function of carbohydrates. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selective formation of glycosidic linkages and the selective reaction of hydroxyl groups; as a result, it relies heavily on the use of protecting groups. Monosaccharides Individual saccharide residues are termed monosaccharides. Carbohydrate synthesis Carbohydrate synthesis is a sub-field of organic chemistry concerned specifically with the generation of natural and unnatural carbohydrate structures. This can include the synthesis of monosaccharide residues or structures containing more than one monosaccharide, known as oligosaccharides. Glycosidic bond formation * Chemical glycosylation * Fischer glycosidation * Glycosyl halide * Koenigs-Knorr reaction Protecting groups * Carbohydrate acetalisation * Trimethylsilyl * Benzyl Ether * para ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs. History In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name ''naphthaline'', as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar). Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two fused benzene rings was proposed by Emil Erlenmeye ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azulene
Azulene is an organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates. Azulene has a long history, dating back to the 15th century as the azure-blue chromophore obtained by steam distillation of German chamomile. The chromophore was discovered in yarrow and wormwood and named in 1863 by Septimus Piesse. Its structure was first reported by Lavoslav Ružička, followed by its organic synthesis in 1937 by Placidus Plattner. Structure and bonding left, The blue color of the mushroom '' Lactarius indigo'' is due to the azulene derivative (7-isopropenyl-4-methylazulen-1-yl)methyl stearate. Azulene is usually viewed as resulting from fusion of cyclopentadiene and cycloheptatrie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |