Thermal Rearrangement Of Aromatic Hydrocarbons on:
[Wikipedia]
[Google]
[Amazon]
 Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (
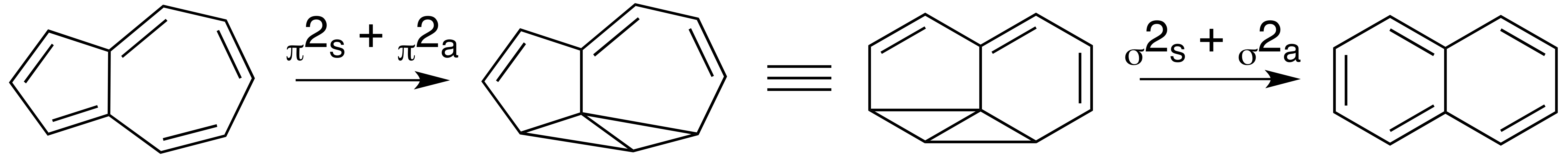 Subsequent work showed that the isomerization of azulene to naphthalene is not readily reversible ( the free energy of a naphthalene to azulene isomerization was too high - approximately 90 kcal/mol). A new reaction mechanism was suggested that involved a
Subsequent work showed that the isomerization of azulene to naphthalene is not readily reversible ( the free energy of a naphthalene to azulene isomerization was too high - approximately 90 kcal/mol). A new reaction mechanism was suggested that involved a
 The diradical mechanism has been supported by kinetic studies performed on the reaction, which have revealed that the reaction is not truly unimolecular, as it is most likely initiated by hydrogen addition from another gas-phase species. However, the reaction still obeys
The diradical mechanism has been supported by kinetic studies performed on the reaction, which have revealed that the reaction is not truly unimolecular, as it is most likely initiated by hydrogen addition from another gas-phase species. However, the reaction still obeys  Benzene ring contractions are the last two mechanisms that have been suggested, and they are currently the preferred mechanisms. These reaction mechanisms proceed through the lowest free energy transition states compared to the diradical and dyotropic mechanisms. The difference between the two ring contractions is minute however, so it has not been determined which is favored over the other. Both mechanisms are shown as follows for the ring contraction of
Benzene ring contractions are the last two mechanisms that have been suggested, and they are currently the preferred mechanisms. These reaction mechanisms proceed through the lowest free energy transition states compared to the diradical and dyotropic mechanisms. The difference between the two ring contractions is minute however, so it has not been determined which is favored over the other. Both mechanisms are shown as follows for the ring contraction of  The first involves a 1,2-hydrogen shift to a carbene followed by a 1,2-carbon shift on the same C-C bond but in opposite directions. The second differs from the first only by the order of the 1,2-shifts, with the 1,2-carbon shift preceding the 1,2-hydrogen shift.
The four described mechanisms would all result in the isomerization from azulene to naphthalene. Kinetic data and 13C-labeling have been used to elucidate the correct mechanism, and have led organic chemists to believe that one of the benzene ring contractions is the most likely mechanism through which these isomerizations of aromatic hydrocarbons occur.
The first involves a 1,2-hydrogen shift to a carbene followed by a 1,2-carbon shift on the same C-C bond but in opposite directions. The second differs from the first only by the order of the 1,2-shifts, with the 1,2-carbon shift preceding the 1,2-hydrogen shift.
The four described mechanisms would all result in the isomerization from azulene to naphthalene. Kinetic data and 13C-labeling have been used to elucidate the correct mechanism, and have led organic chemists to believe that one of the benzene ring contractions is the most likely mechanism through which these isomerizations of aromatic hydrocarbons occur.
 The oldest characterized thermal rearrangement of an aromatic compound was that of the isomerization of azulene to naphthalene by Heilbronner et al. in 1947.Heilbronner, E.; Plattner, P. A.; Wieland, K. Rearrangement of Azulene to Naphthalene. ''Experientia'' 1947, 3, 70–71. Since then, many other isomerizations have been recorded, however the rearrangement of azulene to naphthalene has received the most attention. Likewise, since the characterization of the automerization of naphthalene by Scott in 1977, similar atom scramblings of other aromatic hydrocarbons such as
The oldest characterized thermal rearrangement of an aromatic compound was that of the isomerization of azulene to naphthalene by Heilbronner et al. in 1947.Heilbronner, E.; Plattner, P. A.; Wieland, K. Rearrangement of Azulene to Naphthalene. ''Experientia'' 1947, 3, 70–71. Since then, many other isomerizations have been recorded, however the rearrangement of azulene to naphthalene has received the most attention. Likewise, since the characterization of the automerization of naphthalene by Scott in 1977, similar atom scramblings of other aromatic hydrocarbons such as
Automerization of Pyrene a Test for the Mechanism of Naphthalene Automerization
''Tetrahedron Letters'' 1982, 23 (18), 1859–1862. DOI: 10.1016/S0040-4039(00)87204-4
Automerizations_in_Benzenoid_Hydrocarbons._New_Mechanistic_Insights_from_the_Thermal_Rearrangement_of_Benz[''a''
/nowiki>anthracene-5-13C.html" ;"title="'a''">Automerizations in Benzenoid Hydrocarbons. New Mechanistic Insights from the Thermal Rearrangement of Benz[''a''
/nowiki>anthracene-5-13C">'a''">Automerizations in Benzenoid Hydrocarbons. New Mechanistic Insights from the Thermal Rearrangement of Benz[''a''
/nowiki>anthracene-5-13C ''Tetrahedron Letters'' 1984, 25 (16), 1661–1664. DOI: 10.1016/S0040-4039(01)81138-2 and even
Formation Mechanism of Polycyclic Aromatic Hydrocarbons and Fullerenes in Premixed Benzene Flames
''Combustion and Flame'' 1999, 119 (1–2), 1–22. DOI: 10.1016/S0010-2180(99)00032-2
 Many of the
Many of the
 Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
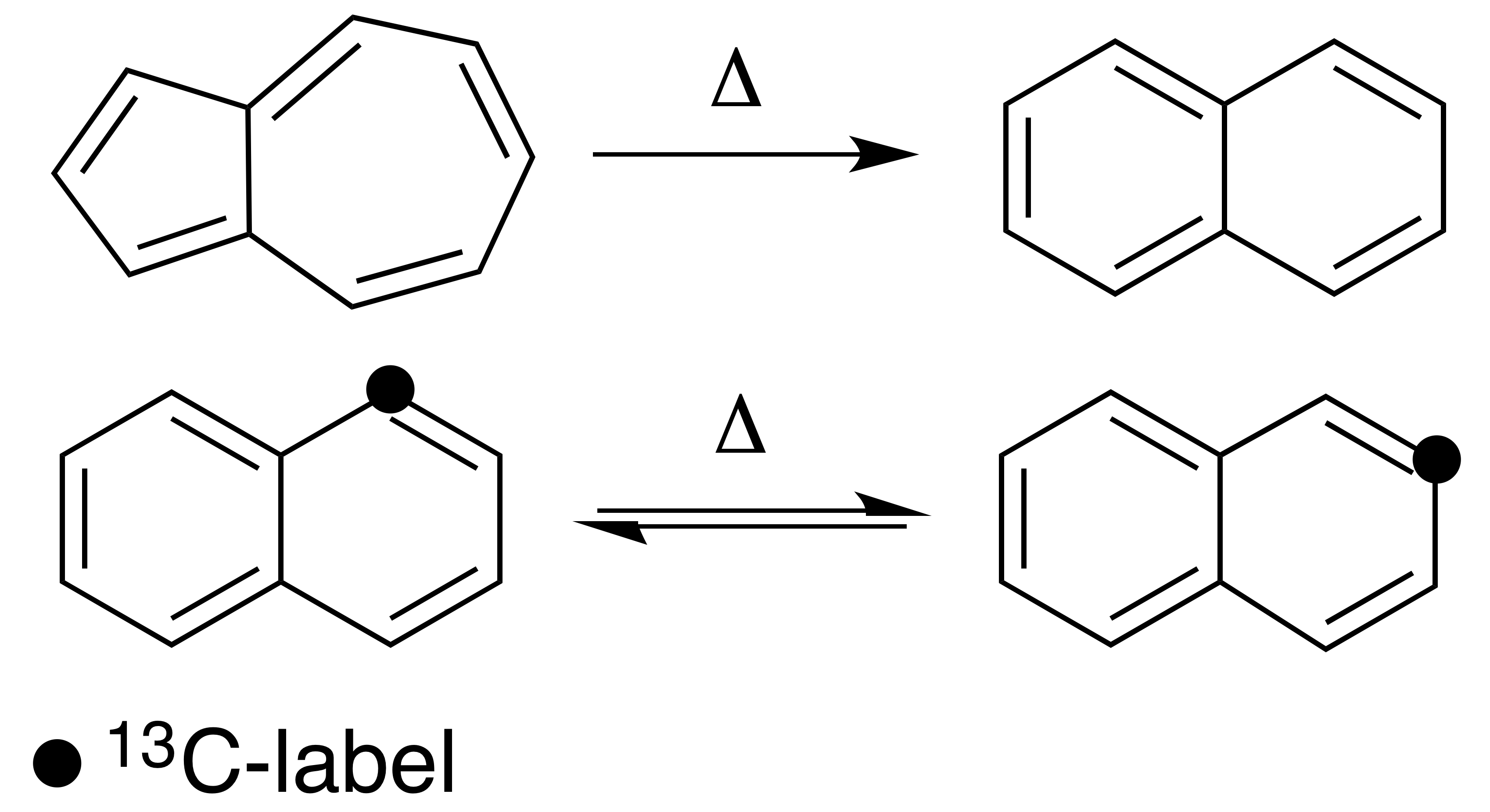
), and one in which the atoms are scrambled but no net change in the aromatic ring occurs ( automerization). The general reaction schemes of the two types are illustrated in Figure 1.
This class of reactions was uncovered through studies on the automerization of naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
as well as the isomerization of unsubstituted azulene
Azulene is an organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature ...
, to naphthalene. Research on thermal rearrangements of aromatic hydrocarbons has since been expanded to isomerizations and automerizations of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
and polycyclic aromatic hydrocarbons
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. P ...
.
Mechanisms
Automerizations
The first proposed mechanism for a thermal rearrangement of an aromatic compound was for the automerization ofnaphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
. It was suggested that the rearrangement of naphthalene occurred due to reversibility of the isomerization of azulene to naphthalene. This mechanism would therefore involve an azulene
Azulene is an organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature ...
intermediate and is depicted below:
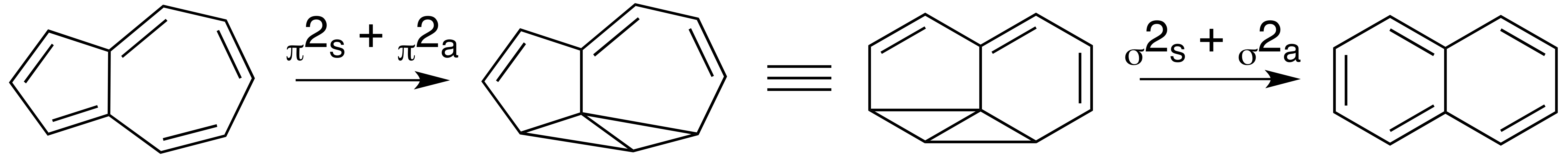 Subsequent work showed that the isomerization of azulene to naphthalene is not readily reversible ( the free energy of a naphthalene to azulene isomerization was too high - approximately 90 kcal/mol). A new reaction mechanism was suggested that involved a
Subsequent work showed that the isomerization of azulene to naphthalene is not readily reversible ( the free energy of a naphthalene to azulene isomerization was too high - approximately 90 kcal/mol). A new reaction mechanism was suggested that involved a carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
intermediate and consecutive 1,2-hydrogen and 1,2-carbon shifts across the same C-C bond but in opposite directions. This is currently the preferred mechanism and is as follows:
Isomerizations
The isomerization of unsubstituted azulene to naphthalene was the first reported thermal transformation of an aromatic hydrocarbon, and has consequently been the most widely studied rearrangement. However, the following mechanisms are generalized to all thermal isomerizations of aromatic hydrocarbons. Many mechanisms have been suggested for this isomerization, yet none have been unequivocally determined as the only correct mechanism. Five mechanisms were originally considered: a reversible ring-closure mechanism, which is shown above, a norcaradiene-vinylidene
Methylidenecarbene (systematically named λ2-ethene and dihydrido-1κ2''H''-dicarbon(''C''—''C'')) is an organic compound with the chemical formula (also written [] or ). It is a metastability, metastable proton tautomer of acetylene, which on ...
mechanism, a diradical mechanism, a methylene walk mechanism, and a spiro compound, spiran mechanism. It was quickly determined that the reversible ring-closure mechanism was inaccurate, and it was later decided that there must be multiple reaction pathways occurring simultaneously. This was widely accepted, as at such high temperatures, one mechanism would have to be substantially energetically favored over the others to be occurring alone. Energetic studies displayed similar activation energies for all possible mechanisms.
Four mechanisms for thermal isomerizations have been proposed: a dyotropic mechanism, a diradical mechanism, and two benzene ring contraction mechanisms; a 1,2-carbon shift to a carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
preceding a 1,2-hydrogen shift, and a 1-2-hydrogen shift to a carbene followed by a 1,2-carbon shift. The dyotropic mechanism involves concerted 1,2-shifts as displayed below. Electronic studies show this mechanism to be unlikely, but it must still be considered a viable mechanism as it has not yet been disproven.
 The diradical mechanism has been supported by kinetic studies performed on the reaction, which have revealed that the reaction is not truly unimolecular, as it is most likely initiated by hydrogen addition from another gas-phase species. However, the reaction still obeys
The diradical mechanism has been supported by kinetic studies performed on the reaction, which have revealed that the reaction is not truly unimolecular, as it is most likely initiated by hydrogen addition from another gas-phase species. However, the reaction still obeys first-order kinetics
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reactio ...
, which is a classical characteristic of radical chain reactions. A mechanistic rational for the thermal rearrangement of azulene to naphthalene is included below. Homolysis of the weakest bond in azulene occurs, followed by a hydrogen shift and ring closure so as to retain the aromaticity of the molecule.
 Benzene ring contractions are the last two mechanisms that have been suggested, and they are currently the preferred mechanisms. These reaction mechanisms proceed through the lowest free energy transition states compared to the diradical and dyotropic mechanisms. The difference between the two ring contractions is minute however, so it has not been determined which is favored over the other. Both mechanisms are shown as follows for the ring contraction of
Benzene ring contractions are the last two mechanisms that have been suggested, and they are currently the preferred mechanisms. These reaction mechanisms proceed through the lowest free energy transition states compared to the diradical and dyotropic mechanisms. The difference between the two ring contractions is minute however, so it has not been determined which is favored over the other. Both mechanisms are shown as follows for the ring contraction of biphenylene
Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon.
Bonding
Biphenylene is a polycyclic h ...
:
 The first involves a 1,2-hydrogen shift to a carbene followed by a 1,2-carbon shift on the same C-C bond but in opposite directions. The second differs from the first only by the order of the 1,2-shifts, with the 1,2-carbon shift preceding the 1,2-hydrogen shift.
The four described mechanisms would all result in the isomerization from azulene to naphthalene. Kinetic data and 13C-labeling have been used to elucidate the correct mechanism, and have led organic chemists to believe that one of the benzene ring contractions is the most likely mechanism through which these isomerizations of aromatic hydrocarbons occur.
The first involves a 1,2-hydrogen shift to a carbene followed by a 1,2-carbon shift on the same C-C bond but in opposite directions. The second differs from the first only by the order of the 1,2-shifts, with the 1,2-carbon shift preceding the 1,2-hydrogen shift.
The four described mechanisms would all result in the isomerization from azulene to naphthalene. Kinetic data and 13C-labeling have been used to elucidate the correct mechanism, and have led organic chemists to believe that one of the benzene ring contractions is the most likely mechanism through which these isomerizations of aromatic hydrocarbons occur.
History
Indications of thermal rearrangements of aromatic hydrocarbons were first noted in the early 20th century bynatural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
s chemists who were working with sesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modificat ...
s. At the time, they noticed the automerization of a substituted azulene shown below, but no further structural or mechanistic investigations were made.
 The oldest characterized thermal rearrangement of an aromatic compound was that of the isomerization of azulene to naphthalene by Heilbronner et al. in 1947.Heilbronner, E.; Plattner, P. A.; Wieland, K. Rearrangement of Azulene to Naphthalene. ''Experientia'' 1947, 3, 70–71. Since then, many other isomerizations have been recorded, however the rearrangement of azulene to naphthalene has received the most attention. Likewise, since the characterization of the automerization of naphthalene by Scott in 1977, similar atom scramblings of other aromatic hydrocarbons such as
The oldest characterized thermal rearrangement of an aromatic compound was that of the isomerization of azulene to naphthalene by Heilbronner et al. in 1947.Heilbronner, E.; Plattner, P. A.; Wieland, K. Rearrangement of Azulene to Naphthalene. ''Experientia'' 1947, 3, 70–71. Since then, many other isomerizations have been recorded, however the rearrangement of azulene to naphthalene has received the most attention. Likewise, since the characterization of the automerization of naphthalene by Scott in 1977, similar atom scramblings of other aromatic hydrocarbons such as pyrene
Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is . This yellow solid is the smallest peri-fused PAH (one where the rings are fused through mor ...
,Scott, L. T.; Kirms, M. A.; Berg, A.; Hansen, P. EAutomerization of Pyrene a Test for the Mechanism of Naphthalene Automerization
''Tetrahedron Letters'' 1982, 23 (18), 1859–1862. DOI: 10.1016/S0040-4039(00)87204-4
azulene
Azulene is an organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature ...
, benz 'a''nthraceneScott, L. T.; Tsang, T.-H.; Levy, L. AAutomerizations_in_Benzenoid_Hydrocarbons._New_Mechanistic_Insights_from_the_Thermal_Rearrangement_of_Benz[''a''
/nowiki>anthracene-5-13C.html" ;"title="'a''
/nowiki>anthracene-5-13C">'a''
/nowiki>anthracene-5-13C ''Tetrahedron Letters'' 1984, 25 (16), 1661–1664. DOI: 10.1016/S0040-4039(01)81138-2
benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
have been described. While the existence of these reactions has been confirmed, the isomerization and automerization mechanisms remain unknown.
Reaction conditions and flash vacuum pyrolysis
Thermal rearrangements of aromatic hydrocarbons are generally carried out through flash vacuum pyrolysis (FVP). In a typical FVP apparatus, a sample is sublimation (phase transition), sublimed under high vacuum (0.1-1.0 millimeter of mercury, mmHg), heated in the range of 500-1100 °C by an electric furnace as it passes through a horizontalquartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical form ...
tube, and collected in a cold trap. Sample is carried through the apparatus by nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
carrier gas.
FVP has numerous limitations:
*First, it requires a slow rate of sublimation to minimize bimolecular reactions in the gas phase
In the outline of physical science, physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, ref ...
, limiting the amount of material that can be reacted in a given amount of time.
*Second, the high temperatures used in FVP often lead to reactant or product degradation. Combined, these first two limitations restrict FVP yields to the range of 25-30%.
*Third, the high temperatures used in FVP do not allow for the presence of functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s, thereby limiting possible products.
*Fourth, as FVP is a gas-phase process, difficulties are frequently encountered when scaling above the milligram level.
*Fifth, the FVP synthesis of strained systems mandates temperatures exceeding 1100 °C, which can lead to the degradation and softening of the expensive quartz apparati.
Possible applications
Thermal rearrangements of aromatic hydrocarbons have been shown to be important in areas of chemical research and industry includingfullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
synthesis, materials applications, and the formation of soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolysed ...
in combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
. Thermal rearrangements of aceanthrylene and acephenanthrylene can yield fluoranthene
Fluoranthene is a polycyclic aromatic hydrocarbon (PAH). The molecule can be viewed as the fusion of naphthalene and benzene unit connected by a five-membered ring. Although samples are often pale yellow, the compound is colorless. It is soluble ...
, an important species in syntheses of corannulene
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20 H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is irculene. It is of scientific interest because it is a geodesi ...
and fullerenes that proceed through additional internal rearrangements.Richter, H.; Grieco, W. J.; Howard, J. BFormation Mechanism of Polycyclic Aromatic Hydrocarbons and Fullerenes in Premixed Benzene Flames
''Combustion and Flame'' 1999, 119 (1–2), 1–22. DOI: 10.1016/S0010-2180(99)00032-2

 Many of the
Many of the polycyclic aromatic hydrocarbons
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. P ...
known to be tumorigenic
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abno ...
or mutagen
In genetics, a mutagen is a physical or chemical agent that permanently changes nucleic acid, genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can ca ...
ic are found in atmospheric aerosol
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be natural or Human impact on the environment, anthropogenic. Examples of natural aerosols are fog o ...
s, which is connected to the thermal rearrangement of polycyclic aromatic hydrocarbons in fast soot formation during combustion.
References
{{reflist Organic chemistry Aromatic hydrocarbons Reaction mechanisms Photochemistry Rearrangement reactions Carbon-carbon bond forming reactions