|
Thermal Rearrangement Of Aromatic Hydrocarbons
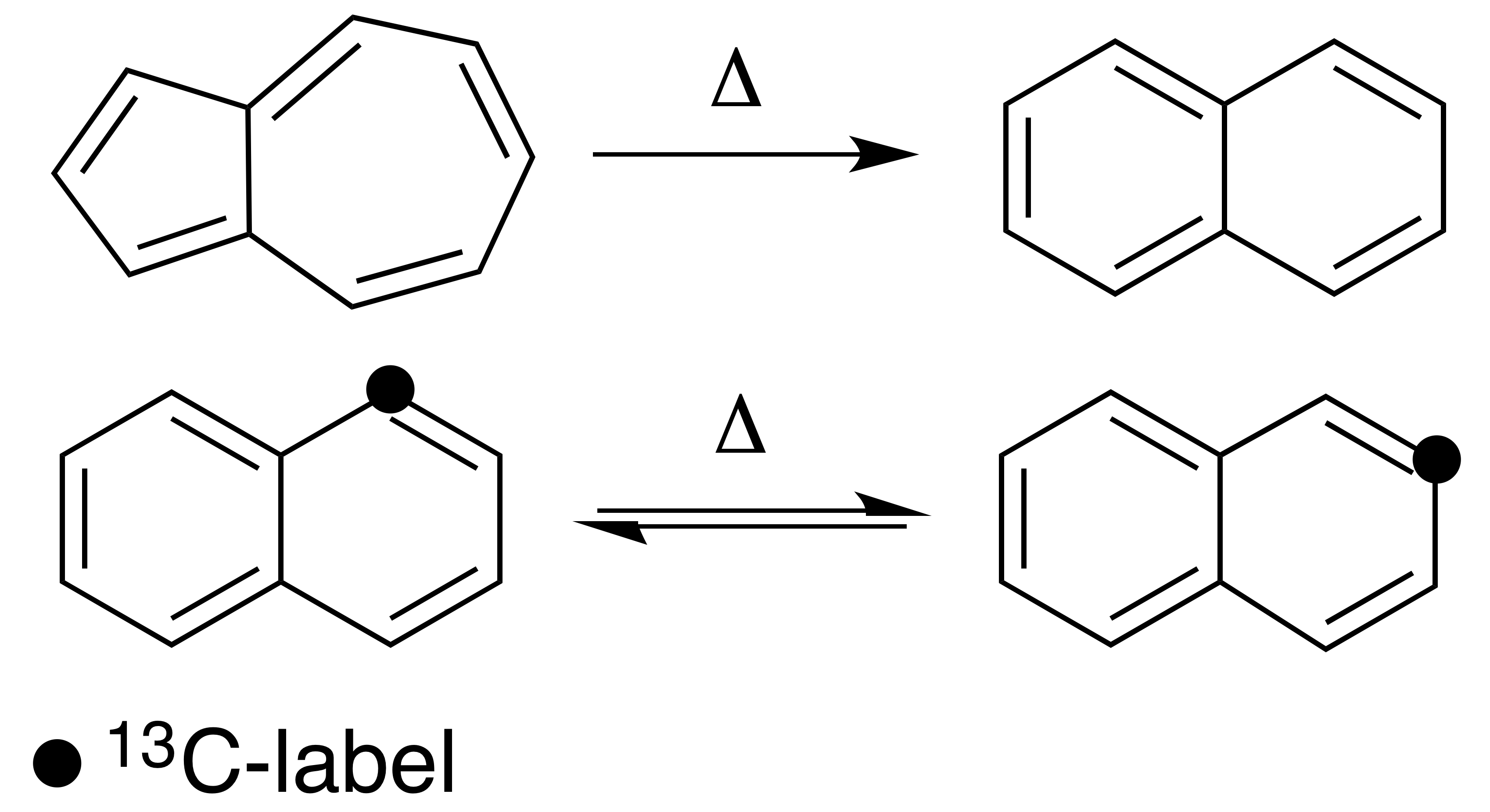
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs ( automerization). The general reaction schemes of the two types are illustrated in Figure 1. This class of reactions was uncovered through studies on the automerization of naphthalene as well as the isomerization of unsubstituted azulene, to naphthalene. Research on thermal rearrangements of aromatic hydrocarbons has since been expanded to isomerizations and automerizations of benzene and polycyclic aromatic hydrocarbons. Mechanisms Automerizations The first proposed mechanism for a thermal rearrangement of an aromatic compound was for the automerization of naphth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerization And Automerization Of Azulene And Naphthalene
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> \ov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diradical
In chemistry, a diradical is a molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactive and in fact rarely isolated. Diradicals are even-electron molecules but have one fewer bond than the number permitted by the octet rule. Examples of diradical species can also be found in coordination chemistry, for example among bis(1,2-dithiolene) metal complexes. Spin states Diradicals are usually triplets. The phrases ''singlet'' and ''triplet'' are derived from the multiplicity of states of diradicals in electron spin resonance: a singlet diradical has one state (S = 0, Ms = 2*0+1 = 1, ms = 0) and exhibits no signal in EPR and a triplet diradical has 3 states (S = 1, Ms = 2*1+1 = 3, ms = -1; 0; 1) and shows in EPR 2 peaks (if no hyperfine splitting). The triplet state has total spin quantum number S = 1 and is paramagnetic. Therefore, di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon And Hydrogen Shifts In Thermal Rearrangements Of Aromatic Hydrocarbons
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. The atoms of carbon can bond t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biphenylene
Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon. Bonding Biphenylene is a polycyclic hydrocarbon, composed of two benzene rings joined by two bridging bonds (as opposed to a normal ring fusion), thus forming a 6-4-6 arene system. The resulting planar structure was one of the first π-electronic hydrocarbon systems discovered to show evidence of antiaromaticity. The spectral and chemical properties show the influence of the central nring, leading to considerable interest in the system in terms of its degree of lessened aromaticity. Questions of bond alternation and ring currents have been investigated repeatedly. Both X-ray diffraction and electron diffraction studies show a considerable alternation of bond lengths, with the bridging bonds between the benzenoid rings having the unusually great length of 1.524 Å. The sepa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Transition
The energy transition is the process of downshifting fossil fuels and re-developing whole systems to operate on low carbon energy sources. More generally, an energy transition is a significant structural change in an energy system regarding supply and consumption. The current transition to sustainable energy is largely driven by a recognition that global greenhouse-gas emissions must be brought to zero. Since fossil fuels are the largest single source of carbon emissions, the quantity that can be produced is limited by the Paris Agreement of 2015 to keep global warming below 1.5 °C. Over 70% of our global greenhouse gas emissions result from the energy sector, for transport, heating, and industrial use. Wind power and solar photovoltaic systems (PV) have the greatest potential to mitigate climate change. Since the late 2010s, the renewable energy transition is also driven by the rapidly increasing competitiveness of both. Another motivation for the transition is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diradical Mechanism For Isomerization Of Azulene To Naphthalene
In chemistry, a diradical is a molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactive and in fact rarely isolated. Diradicals are even-electron molecules but have one fewer bond than the number permitted by the octet rule. Examples of diradical species can also be found in coordination chemistry, for example among bis(1,2-dithiolene) metal complexes. Spin states Diradicals are usually triplets. The phrases ''singlet'' and ''triplet'' are derived from the multiplicity of states of diradicals in electron spin resonance: a singlet diradical has one state (S = 0, Ms = 2*0+1 = 1, ms = 0) and exhibits no signal in EPR and a triplet diradical has 3 states (S = 1, Ms = 2*1+1 = 3, ms = -1; 0; 1) and shows in EPR 2 peaks (if no hyperfine splitting). The triplet state has total spin quantum number S = 1 and is paramagnetic. Therefore, di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homolysis (chemistry)
In chemistry, homolysis () or homolytic fission is the dissociation of a molecular bond by a process where each of the fragments (an atom or molecule) retains one of the originally bonded electrons. During homolytic fission of a neutral molecule with an even number of electrons, two free radicals will be generated. That is, the two electrons involved in the original bond are distributed between the two fragment species. Bond cleavage is also possible by a process called heterolysis. The energy involved in this process is called bond dissociation energy (BDE). BDE is defined as the "enthalpy (per mole) required to break a given bond of some specific molecular entity by homolysis," symbolized as ''D''. BDE is dependent on the strength of the bond, which is determined by factors relating to the stability of the resulting radical species. Because of the relatively high energy required to break bonds in this manner, homolysis occurs primarily under certain circumstances: * Light ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free-radical Reaction
A free-radical reaction is any chemical reaction involving free radicals. This reaction type is abundant in organic reactions. Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical by Moses Gomberg (1900) and the lead-mirror experiment described by Friedrich Paneth in 1927. In this last experiment tetramethyllead is decomposed at elevated temperatures to methyl radicals and elemental lead in a quartz tube. The gaseous methyl radicals are moved to another part of the chamber in a carrier gas where they react with lead in a mirror film which slowly disappears. When radical reactions are part of organic synthesis the radicals are often generated from radical initiators such as peroxides or azobis compounds. Many radical reactions are chain reactions with a chain initiation step, a chain propagation step and a chain termination step. Reaction inhibitors slow down a radical reaction and radical disproportionation is a competing reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Order Of Reaction
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders). For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and express the concentration of the species and usually in moles per liter (molarity, ). The exponents and are the partial ''orders of reaction'' for and and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The constant is the reaction rate constant or ''rate coefficient'' of the reaction. Its value may depend on conditions such as temperature, ionic strength, surface area of an adsorbent, or light irradiation. If the reaction goes to completion, the rate equation for the reaction rate v\; =\; k cex cey applies throug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.C.M. Guldberg and P. Waage,"Studies Concerning Affinity" ''Forhandlinger i Videnskabs-Selskabet i Christiania'' (1864), 35P. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concerted Reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not to depend on solvent polarity ruling out large buildup of charge in the transition state. The reaction is said to progress through a concerted mechanism as all bonds are formed and broken ''in concert''. Pericyclic reactions, the S2 reaction, and some rearrangements - such as the Claisen rearrangement - are concerted reactions. The rate of the SN2 reaction is second order overall due to the reaction being bimolecular (i.e. there are two molecular species involved in the rate-determining step). The reaction does not have any intermediate steps, only a transition state In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |