Kalkitoxin on:
[Wikipedia]
[Google]
[Amazon]
Kalkitoxin, a
 Kalkitoxin induces delayed neuronal necrosis in cerebellar
Kalkitoxin induces delayed neuronal necrosis in cerebellar
toxin
A toxin is a naturally occurring organic poison produced by metabolic activities of living cells or organisms. Toxins occur especially as a protein or conjugated protein. The term toxin was first used by organic chemist Ludwig Brieger (1849 ...
derived from the cyanobacterium
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blu ...
''Lyngbya majuscula
''Lyngbya majuscula'' is a species of filamentous cyanobacteria in the genus ''Lyngbya''. It is named after the Dane Hans Christian Lyngbye.
As a result of recent genetic analyses, several new genera were erected from the genus ''Lyngbya'': '' ...
'', induces NMDA receptor mediated neuronal necrosis, blocks voltage-dependent sodium channels, and induces cellular hypoxia by inhibiting the electron transport chain (ETC) complex 1.
Natural sources
Kalkitoxin is anichthyotoxin Ichthyotoxins are compounds which are either toxic to fish, or are toxins produced by fish. The former include the algae-produced euglenophycin and prymnesins, which can cause large-scale fish deaths. The latter includes ostracitoxin, produced by ...
, derived from the cyanobacterium
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blu ...
''Lyngbya majuscula
''Lyngbya majuscula'' is a species of filamentous cyanobacteria in the genus ''Lyngbya''. It is named after the Dane Hans Christian Lyngbye.
As a result of recent genetic analyses, several new genera were erected from the genus ''Lyngbya'': '' ...
'' which covers sections of the coral reef. It typically forms mini-blooms and produces several metabolites, such as kalkitoxin, curacin-A and antillatoxin. Kalkitoxin has been found and purified near the coasts of Curaçao and Puerto Rico.
Structure and reactivity
Kalkitoxin is alipopeptide
A lipopeptide is a molecule consisting of a lipid connected to a peptide. They are able to self-assemble into different structures. Many bacteria produced these molecules as a part of their metabolism, especially those of the genus ''Bacillus'', ' ...
toxin with a molecular weight of 366.604Da. Its chemical formula is C21H38N2OS. The structure contains two double bonds, a 2,4-disubstituted thiazoline ring system, and an additional carbonyl-group. These four groups each provide a degree of unsaturation, which causes kalkitoxin to have four degrees of unsaturation
In the analysis of the molecular formula of organic molecules, the degree of unsaturation (also known as the index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index) is a calculation that determines the total number of r ...
. The structure contains 5 chiral centers
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups cr ...
, one of which is due to a substituent of the thiazoline ring, and the other four are due to methine group
In organic chemistry, a methine group or methine bridge is a trivalent functional group , derived formally from methane. It consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen. T ...
s along the aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, ...
carbon chain, which are tertiary carbon atoms bearing three single carbon bonds and one hydrogen. The four methyl groups (each at a methine chiral center), the structure's overall stereochemistry, and the N-methyl group all contribute to the toxicity of kalkitoxin.

Structure determination
The structure of kalkitoxin was first determined by characterizing six partial structures which were subsequently connected to yield the total structure. This investigation was largely carried out through variousNMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
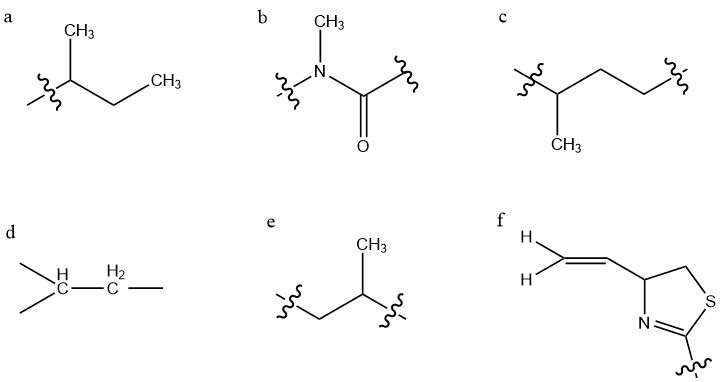
experiments. Structure (a) is a sec-butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, g ...
group, indicated by characteristic deshielding of its central methine group
In organic chemistry, a methine group or methine bridge is a trivalent functional group , derived formally from methane. It consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen. T ...
due to the adjacent carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
. Structure (b) contains this carbonyl group, and an adjacent tertiary methylated nitrogen atom, constituting a tertiary amide group. Since this is a tertiary amide, it exists in a cis/trans mixture, which underlies the two conformations of kalkitoxin. Structure (c) is a string of two methylene groups, then a methine group bearing a high-field methyl group. The next two groups identified (d,e) are identical and opposing strings of CH2-CH-CH3, however the left grouping's methylene protons experience greater deshielding, due to their proximity to the adjacent imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
. Deshielding is an effect of a nearby electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
atom withdrawing electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
from a given atom nucleus, eliciting an increased chemical shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure o ...
as measured by NMR.
The final partial structure consists of a thiazoline
Thiazolines (or dihydrothiazoles) are a group of isomeric 5-membered heterocyclic compounds containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivatives are more comm ...
ring with a terminal alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
substituent, as determined by electron ionization mass spectrometry (EI-MS) and 13C NMR. The chemical shifts of ring carbons adjacent to the sulfur and nitrogen heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecula ...
s were compared to 13C NMR data from model compounds. This allowed for the determination of these heteroatoms' locations in the ring, and subsequently the existence of the thiazoline ring itself. With these partial structures established, their connectivity was evaluated via HMBC spectroscopy, a 2D NMR technique which allows for the determination of heteronuclear J-coupling
In nuclear chemistry and nuclear physics, ''J''-couplings (also called spin-spin coupling or indirect dipole–dipole coupling) are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that ...
values for nonadjacent carbons and protons. This allows for the spatial relation of specific carbon and hydrogen atoms within a structure to be determined.
Stereochemistry
Kalkitoxin has fivechiral centers
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups cr ...
, one of which is the ring carbon to which the terminal alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
is coordinated, with the remaining four occurring at tertiary carbon atoms along the aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, ...
chain originating from the imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
nitrogen. The total stereochemistry of natural (+)-kalkitoxin is 3R,7R,8S,10S,2′R. For this determination, 3JCH values by a variation of the HSQMBC pulse technique, a type of HMBC spectroscopy, and 3JHH values by exclusive correlation spectroscopy
Exclusive correlation spectroscopy (ECOSY) is an NMR Correlation spectroscopy, correlation experiment introduced by O. W. Sørensen, Christian Griesinger, Richard R. Ernst and coworkers for the accurate measurement of small J-couplings.
The idea ...
(E.COSY). These methods use NMR to evaluate the spin-spin coupling constants which directly relate to the dihedral angle
A dihedral angle is the angle between two intersecting planes or half-planes. In chemistry, it is the clockwise angle between half-planes through two sets of three atoms, having two atoms in common. In solid geometry, it is defined as the un ...
of the atoms being analyzed, allowing for the determination of chirality. This was used to determine the stereochemistry of chiral centers at C7, C8, and C10. Because C7 and C8 are adjacent stereocenters, these techniques allowed for immediate determination of their relative stereochemistry, however C10 is separated from C8 by C9, which carries two diastereotopic protons. This allows for the determination of relative stereochemistry of C8 and C10 to the C9 protons through J-coupling \ 3J coupling values, so as to relate the relative stereochemistry of C8 to C10. These methods yielded a relative stereochemistry of 7R, 8S, 10S for the aliphatic chain stereocenters. Stereochemistry at C3 was determined by Marfey's analysis, wherein the compound was ozonized and subsequently hydrolyzed to obtain cysteic acid
Cysteic acid also known as 3-sulfo--alanine is the organic compound with the formula HO3SCH2CH(NH2)CO2H. It is often referred to as cysteate, which near neutral pH takes the form −O3SCH2CH(NH3+)CO2−.
It is an amino acid generated by oxidation ...
from the thiazoline ring and attached terminal alkene. Marfey's analysis indicated this amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
derivative was L-cysteic acid, indicating R absolute stereochemistry at C3. The absolute stereochemistry of the total molecule was determined by synthesizing the possible configurations of the already determined relative chiralities, and comparison of these to natural Kalkitoxin via 13C NMR shift differences, revealing the natural (+)-kalkitoxin stereochemistry to be 3R,7R,8S,10S,2′R.
Structure-activity relationship
The structure-activity relationship (SAR) of a molecule is the connection between the structural moieties within the compound, and how those specific structures directly contribute to the extent and character of the molecule's biological activity. Kalkitoxin exhibits potent cytotoxicity which relies on the completethiazoline
Thiazolines (or dihydrothiazoles) are a group of isomeric 5-membered heterocyclic compounds containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivatives are more comm ...
ring for its action. Kalkitoxin analogs lacking the complete thiazoline ring exhibit on the order of 1000-fold decreased toxicity to solid tumor cell lines. This indicates the thiazoline ring structure is a crucial component of kalkitoxin's mechanism of cytotoxicity. The necessity of the stereochemistry exhibited in natural (+)-kalkitoxin decreases moving towards the chiral centers in the core of the molecule, while the more terminal chiral centers and amide methyl group are increasingly crucial for toxicity. In a study which assayed for the toxicity of kalkitoxin and various analogs against brine shrimp, the analogs which experienced the least significant loss of potency were epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization i ...
s at either C8 or C10. This indicates that C8 and C10 chiralities in natural (+)-kalkitoxin are the least critical for toxic biological activity. It is apparent that C10 chirality is less critical than C8, because the epimer of (+)-kalkitoxin at C10 is more potent than the epimer at C8. Furthermore, the removal of the C10 methyl group has a smaller impact on potency than does epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization i ...
ization of C7, supporting the trend of decreased SAR correlation at core chiral centers on the aliphatic chain. Epimerization at C3, the attachment point of the terminal alkene to the thiazoline ring, further decreases potency of kalkitoxin, in agreement with the thiazoline ring and overall conformation of the leftmost segment of the molecule being critical for bioactivity. Finally, replacement of the tertiary amide with a secondary amide eliminates any observable toxicity, so this structure is crucial in the mechanism of kalkitoxin toxicity.
Synthesis
Wu ''et al.'' synthesis
This effort was the first total synthesis of (+)-kalkitoxin, and served the purpose of deducing the specific stereochemistry of natural kalkitoxin. This synthesis began from an alcohol bearing the proper chirality at C8 and C10 found in (+)-kalkitoxin, and carried a dimethylphenylsiloxy (DPSO) group positioned beta to C8, and a terminalalkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
positioned alpha to C10. Hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration p ...
of this alkene gives the resulting alcohol, which is converted to an azide, which is the position at which (R)-2-methylbutyric acid is coupled to produce the sec-butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, g ...
group and amide group. The amide is subsequently methylated
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These ...
, finalizing the tertiary amide which has been shown to be so crucial for kalkitoxin toxicity. O-Desilylation and oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of the resulting alcohol produce an acceptor for a Horner-Wadsworth-Emmons reaction, wherein a carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
and an alpha-methylated
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These ...
phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing groups (where R = alkyl, aryl, or just hydrogen). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly ...
react to produce an alkene. In this case, a beta-keto phosphonate bearing an (R)-phenylglycine
Phenylglycine is the organic compound with the formula C6H5CH(NH2)CO2H. It is a non-proteinogenic alpha amino acid related to alanine, but with a phenyl group in place of the methyl group. It is a white solid. The compound exhibits some biolog ...
-derived auxiliary
Auxiliary may refer to:
* A backup site or system
In language
* Auxiliary language (disambiguation)
* Auxiliary verb
In military and law enforcement
* Auxiliary police
* Auxiliaries, civilians or quasi-military personnel who provide support of ...
group was ligated to the molecule. This group is lost in asymmetric conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polari ...
of an (R)-amino alcohol, which, through two cyclodehydration steps using Wipf's oxazoline-thiazoline interconversion protocol, produces the thiazoline ring.
White ''et al.'' synthesis
The second total synthesis of (+)-kalkitoxin was only 16 steps and gave a 3% overall yield. A major aspect by which this differs from the first total synthesis of (+)-kalkitoxin is that rather than using a Horner-Wadsworth-Emmons reaction to ligate aphosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing groups (where R = alkyl, aryl, or just hydrogen). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly ...
carrying the 4-phenyl-2-oxazolidinone, an organocopper conjugate addition reaction was used instead. This was done specifically by connecting the organocopper species to a 4-phenyl-2-oxazolidinone carrying an (S)-N-trans- crotonyl group through a 1,4- nucleophilic addition to the α,β-unsaturation of the crotonyl group. This method is advantageous because it allows for stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
of the resulting 1,3-dimethyl configuration during the larger sequential introduction of the methyl substituents at the C7, C8 and C10 chiral centers.
Another point of diversion between these two syntheses is the number of carbons separating the keto-auxiliary group from the chiral center at C7. This group was separated by one carbon from C7 in the first total synthesis, so the keto-auxiliary moiety could be converted to a carboxylic acid, in anticipation of addition of the amino alcohol immediately thereafter. In this synthesis, this keto-auxiliary group is directly adjacent to C7, necessitating a one carbon homologation, before construction of the thiazoline ring. This was achieved through reductive bond cleavage of the auxiliary group to a primary alcohol and oxidation to the corresponding aldehyde, Wittig reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Mos ...
using an ylide carrying a methoxy group to produce an enol ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers includ ...
, hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
to the aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
and finally oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
to produce the carboxylic acid.
Balieu ''et al.'' synthesis
This synthesis differentiates itself in that it takes an "assembly line
An assembly line is a manufacturing process (often called a ''progressive assembly'') in which parts (usually interchangeable parts) are added as the semi-finished assembly moves from workstation to workstation where the parts are added in se ...
" synthesis approach, as opposed to the conventional iterative synthetic approach taken in previous syntheses which normally necessitate functional-group interconversions and repetitive purifications for aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, ...
chain extensions, such as the one found in kalkitoxin. This novel approach is achieved through the use of reagent-controlled chain extension of a boronic ester, which relies on a spontaneous 1,2-migration after formation of an intermediate compound incorporating a newly added lithiated benzoate
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, wh ...
ester building block.
This allows for control of chirality at each addition by selecting the chirality of each benzoate ester added. Furthermore, this avoids repetitive interconversion and purification steps normally required for repeat chain extensions, which increases yield and efficiency and decreases labor. This synthesis capitalized on this technique by producing the core aliphatic chain as a single large fragment, and coupled this fragment to the chiral sec-butyl group bearing a carboxylic acid. The opposing amino thioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A su ...
fragment was synthesized separately, and then adjoined and subsequently cyclized following the procedure devised by White et al. In total, this synthesis requires only 7 steps if the initial homologation series is counted as one step.
Targets
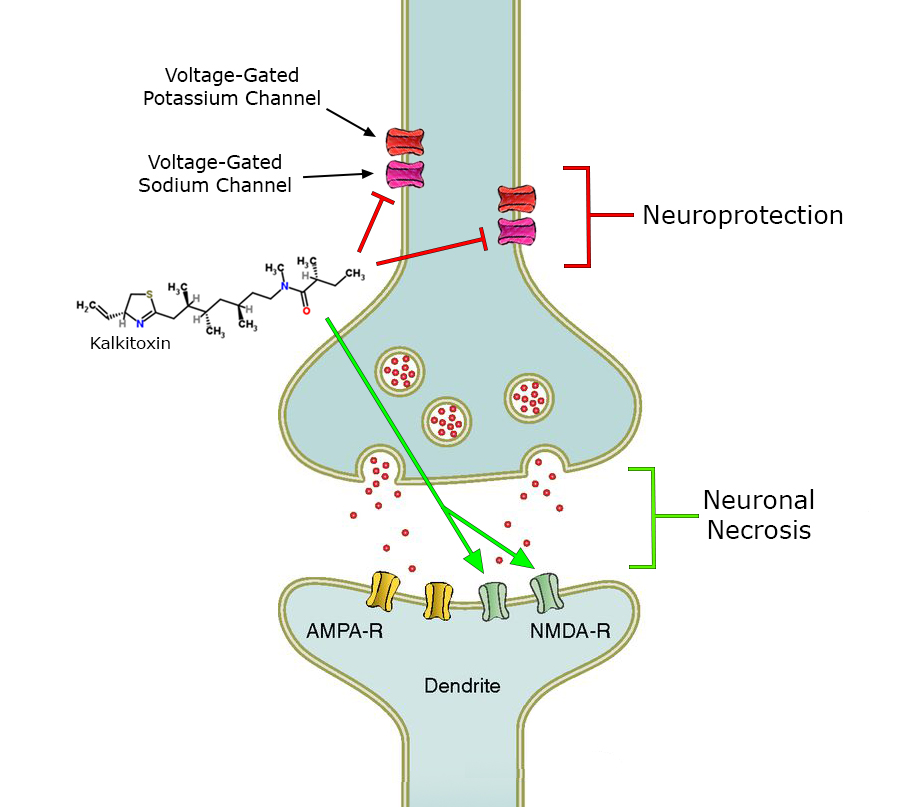
Kalkitoxin may activate the NMDA receptor. It also blocks thevoltage-gated sodium channel
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels and can be classified according to the trigger that opens the channel ...
and the electron transport chain (ETC) complex 1. It remains unknown how exactly kalkitoxin binds to the voltage-gated sodium channel. Neurotoxin sit 1 and 2 have been ruled out as possible binding sites, whereas neurotoxin site 7 is suggested as binding site for kalkitoxin. This is probable, because there is inhibition of the channel by kalkitoxin when deltamethrin, which has positive allosteric effects, is present. This could be because molecular determinants for binding are similar in kalkitoxin and deltamethrin.
Mode of action
 Kalkitoxin induces delayed neuronal necrosis in cerebellar
Kalkitoxin induces delayed neuronal necrosis in cerebellar granule cell
A granule is a large particle or grain. It can refer to:
* Granule (cell biology), any of several submicroscopic structures, some with explicable origins, others noted only as cell type-specific features of unknown function
** Azurophilic granul ...
s of the rat. This neuronal necrosis proved to be NMDA-receptor
The ''N''-methyl-D-aspartate receptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA an ...
mediated. These receptors are normally activated by glutamate and other excitotoxic compounds and can induce neuronal necrosis. It is not yet known if the toxin induces necrosis directly or via the release of excitotoxic compounds.
Secondly, kalkitoxin blocks voltage-gated sodium channels
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels and can be classified according to the trigger that opens the chann ...
, thereby inhibiting Ca2+ release that normally occurs when the voltage-gated sodium channel is activated, in a concentration dependent matter. Calcium release has been shown to induce lactate dehydrogenase
Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of lactate to pyruvate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from one ...
(LDH) production. The amount of LDH is a measure for neuronal cell death. In the presence of kalkitoxin there is also a concentration-dependent inhibition of neuronal cell death and LDH production (9). The mechanism behind this inhibition is still unknown.
Thirdly, kalkitoxin blocks the electron transport chain (ETC) complex 1, one of the protein complexes involved in mitochondrial respiration. By blocking the ETC complex 1, kalkitoxin potently inhibits hypoxia-inducible factor
Hypoxia-inducible factors (HIFs) are transcription factors that respond to decreases in available oxygen in the cellular environment, or hypoxia. They are only present in parahoxozoan animals.
Discovery
The HIF transcriptional complex w ...
-1 (HIF-1) activation. HIF-1 is a transcription factor, which enhances the expression of genes that increase oxygen availability, as well as genes that decrease oxygen consumption. Inhibition of HIF-1, which is one of the main effects of kalkitoxin, thus induces cellular hypoxia.
Toxicity
Kalkitoxin is ichthyotoxic to goldfish (''Carassius auratus
The goldfish (''Carassius auratus'') is a freshwater fish in the family Cyprinidae of order Cypriniformes. It is commonly kept as a pet in indoor aquariums, and is one of the most popular aquarium fish. Goldfish released into the wild have ...
'', LC50: 700nM) and to aquatic crustacean brine shrimp (''Artemia salina
''Artemia salina'' is a species of brine shrimp – aquatic crustaceans that are more closely related to '' Triops'' and cladocerans than to true shrimp. It belongs to a lineage that does not appear to have changed much in .
''A. salina'' is na ...
'', LC50: 150-180nM ). Kalkitoxin also has been shown to have delayed neurotoxic effects on cerebellar granule cells of the rat (LC50: 3,86nM).
Therapeutic research
Many efforts to discover cancer therapeutic drugs focus on the screening of novel biomolecules produced and isolated from various plants and animals. These isolated molecules are screened via in-vitro assays to measure their effects in standardized paradigms designed to select for the desired therapeutic effect. Kalkitoxin was originally isolated fromLyngbya majuscula
''Lyngbya majuscula'' is a species of filamentous cyanobacteria in the genus ''Lyngbya''. It is named after the Dane Hans Christian Lyngbye.
As a result of recent genetic analyses, several new genera were erected from the genus ''Lyngbya'': '' ...
as an effort to collect new molecules for testing as antitumor or antifungal agents. One of the first tests of kalkitoxin tumor-selective cytotoxicity used an in-vitro assay to test solid tumor selectivity of kalkitoxin's previously demonstrated cytotoxicity against the human colon cell line HCT-116. The assay measured the extent of differential cytotoxicity of kalkitoxin and various analogous structures by observing differential cytotoxicity against solid tumor cells, and either non-solid tumor cells such as leukemia
Leukemia ( also spelled leukaemia and pronounced ) is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or ...
cells or normal cells. This test yielded promising results, as kalkitoxin exhibited preferential cytotoxicity for the solid tumor cell test conditions (Colon 38, and HCT-116 cells) as compared to the non-solid tumor and normal cell conditions.
Kalkitoxin exerts this cytotoxic effect through inhibition of the mitochondrial electron transport chain complex 1. This causes the inhibition of hypoxia-induced HIF-1
Hypoxia-inducible factors (HIFs) are transcription factors that respond to decreases in available oxygen in the cellular environment, or hypoxia. They are only present in parahoxozoan animals.
Discovery
The HIF transcriptional complex w ...
activation, which is crucial in solid tumor cancers because hypoxia drives tumor angiogenesis, leading to worsening disease stages and increased resistance to treatment. HIF-1 is a transcription factor
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The f ...
which induces expression of genes promoting oxygen availability and decreasing oxygen consumption, the effect of which counteracts cellular hypoxia.Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. Therefore, kalkitoxin's HIF-1 inhibitory ability positions it as a potentially promising molecule to counteract the progression of some solid tumor cancers by blocking the tumor proliferative response to hypoxia. The caveat to kalkitoxin's promising anti-proliferative properties is its neurotoxic effects. At concentrations comparable to those required for tumor-selective cytotoxicity, kalkitoxin induces cell death when applied to rat cerebellar granule neurons (CGN) in culture. Kalkitoxin acts as an N-methyl-D-aspartate
''N''-methyl--aspartic acid or ''N''-methyl--aspartate (NMDA) is an amino acid derivative that acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor. Unl ...
(NMDA) receptor
Receptor may refer to:
* Sensory receptor, in physiology, any structure which, on receiving environmental stimuli, produces an informative nerve impulse
*Receptor (biochemistry), in biochemistry, a protein molecule that receives and responds to a ...
agonist, and induces cytotoxicity in cultured rat CGNs at delayed time points. Therefore, this effect must be taken into account when considering kalkitoxin or its chemical derivatives for use as a therapeutic option.
References