|
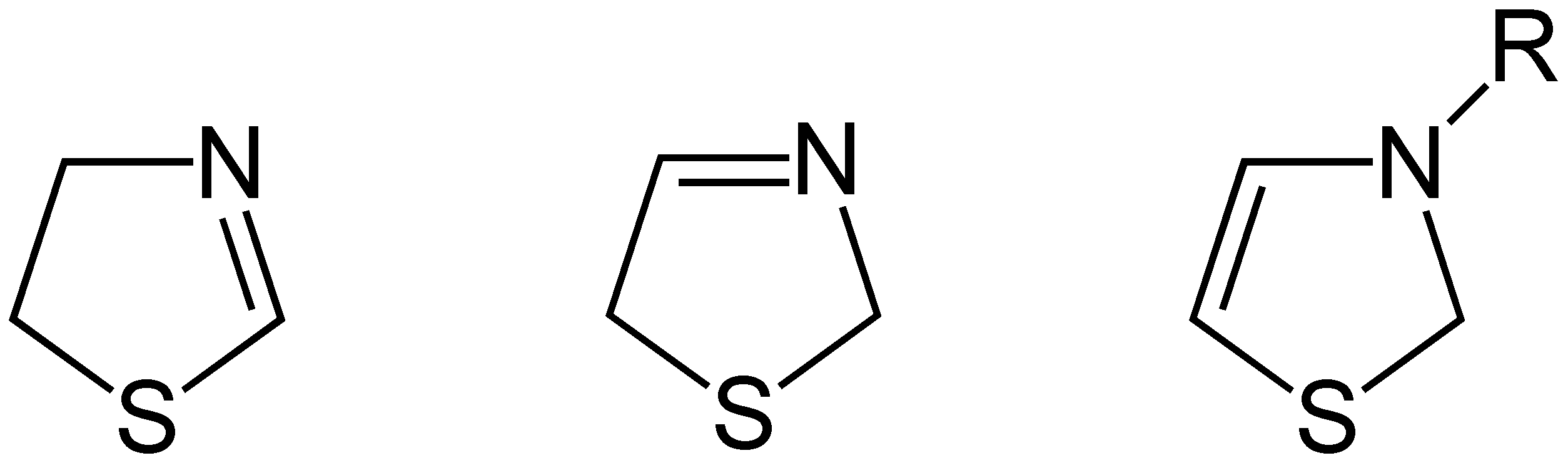
Thiazoline
Thiazolines (or dihydrothiazoles) are a group of isomeric 5-membered heterocyclic compounds containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivatives are more common and some are bioactive. For example, in a common post-translational modification, cysteine residues are converted into thiazolines. The name thiazoline originates from the Hantzsch–Widman nomenclature. Isomers Three structural isomers of thiazoline exist depending on the position of the double bond. These forms do not readily interconvert and hence are not tautomers. Of these 2-thiazoline is the most common. A fourth structure exists in which the N and S atoms are adjacent; this known as isothiazoline. Synthesis Thiazolines were first prepared by dialkylation of thioamides by Richard Willstatter in 1909. 2-Thiazolines are commonly prepared from 2-aminoethanethiols (e.g. cysteamine). They may also be synthesized via the Asinger r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond. Oxazoline itself has no applications however oxazolines have been widely investigated for potential applications. These applications include use as ligands in asymmetric catalysis, as protecting groups for carboxylic acids and increasingly as monomers for the production of polymers. Isomers Synthesis The synthesis of 2-oxazoline rings is well established and in general proceeds via the cyclisation of a 2-amino alcohol (typically obtained by the reduction of an amino acid) with a suitable functional group. The overall mechanism is usuall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazolidine
Thiazolidine is a heterocyclic organic compound with the formula (CH2)3(NH)S. It is a 5-membered saturated ring with a thioether group and an amine group in the 1 and 3 positions. It is a sulfur analog of oxazolidine. Thiazolidine is a colorless liquid. Derivatives, thiazolidines, are known. For example, the drug pioglitazone contains a thiazolidine ring. Another drug that contains a thiazolidine ring is the antibiotic penicillin. Preparation Thiazolidine is prepared as it was in its first reported synthesis, by the condensation of cysteamine and formaldehyde. Other thiazolidines may be synthesized by similar condensations. A notable derivative is 4-carboxythiazolidine, derived from formaldehyde and cysteine. Derivatives N-Methyl-2-thiazolidinethione is an accelerator for the vulcanization of chloroprene rubbers. Thiazolidines functionalized with carbonyls at the 2 and 4 positions, the thiazolidinediones, are drugs used in the treatment of diabetes mellitus type 2. Rhodanin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Aminothiazoline-4-carboxylic Acid
2-Aminothiazoline-4-carboxylic acid (ACTA) is the organosulfur compound and a heterocycle with the formula HO2CCHCH2SCNH2N. This derivative of thiazoline is an intermediate in the industrial synthesis of L-cysteine, an amino acid. ACTA exists in equilibrium with its tautomer 2-iminothiazolidine-4-carboxylic acid. It is produced by the reaction of methyl chloroacrylate with thiourea. It is also a biomarker for cyanide poisoning, as it results from the condensation of cysteine and cyanide Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms. In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of .... References {{DEFAULTSORT:Aminothiazoline-4-carboxylic acid, 2- Thiazolines Carboxylic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Firefly Luciferin
Firefly luciferin (also known as beetle luciferin) is the luciferin, or light-emitting compound, used for the firefly (Lampyridae), railroad worm (Phengodidae), starworm (Rhagophthalmidae), and click-beetle (Pyrophorini) bioluminescent systems. It is the substrate of luciferase (Enzyme Commission number, EC 1.13.12.7), which is responsible for the characteristic yellow light emission from many firefly species. As with all other luciferins, oxygen is required to elicit light; however, it has also been found adenosine triphosphate (ATP) and magnesium are required for light emission. History Much of the early work on the chemistry of the firefly luminescence was done in the lab of William D. McElroy at Johns Hopkins University. The luciferin was first isolated and purified in 1949, though it would be several years until a procedure was developed to crystallize the compound in high yield. This, along with the synthesis and structure elucidation, was accomplished by Dr. Emil H. White ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group. Oxazoles are related compounds, with sulfur replaced by oxygen. Thiazoles are structurally similar to imidazoles, with the thiazole sulfur replaced by nitrogen. Thiazole rings are planar and aromatic. Thiazoles are characterized by larger pi-electron delocalization than the corresponding oxazoles and have therefore greater aromaticity. This aromaticity is evidenced by the chemical shift of the ring protons in proton NMR spectroscopy (between 7.27 and 8.77 ppm), clearly indicating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fireflies
The Lampyridae are a family (biology), family of Elateroidea, elateroid beetles with more than 2,000 described species, many of which are bioluminescence, light-emitting. They are soft-bodied beetles commonly called fireflies, lightning bugs, or glowworms for their conspicuous production of light, mainly crepuscular, during twilight, to attract mates. Light production in the Lampyridae is thought to have originated as an honest signal, honest Aposematism, warning signal that the larvae were distasteful; this was co-opted in evolution as a mating signal in the adults. In a further development, female fireflies of the genus ''Photuris'' mimic the flash pattern of ''Photinus (beetle), Photinus'' species to trap their males as prey. Fireflies are found in temperate and tropical climates. Many live in marshes or in wet, wooded areas where their larvae have abundant sources of food. While all known fireflies glow as larvae, only some species produce light in their adult stage, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asinger Reaction
The Asinger-reaction (sometimes referred to as the Asinger-4 component reaction or A-4CR for short) is a multicomponent reaction for the synthesis of 3-thiazolines and other related heterocycles. It is named after Friedrich Asinger who first reported it in 1956. Process An α-halogenated carbonyl-component reacts with sodium hydrosulfide (NaSH) and forms a Thiol ''in situ''. The thiol reacts directly with another carbonyl component and ammonia to form a thiazoline. The reaction works also by using elemental sulphur, an α–substituted ketone, another carbonyl component and ammonia; in this case, a mixture of products is formed. The formation of 3-thiazolines also occurs by using α-thioaldehyde or α-thioketone and ammonia. A simplified route of the Asinger-reaction was developed at Degussa. An α-halogenated carbonyl compound reacts with sodium hydrosulfide (NaSH) and forms a Thiol ''in situ'' which reacts directly with aldehydes or ketones and ammonia to 3-thiazolines. The ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteamine
Cysteamine is a chemical compound that can be biosynthesized in mammals, including humans, by the degradation of coenzyme A. The intermediate pantetheine is broken down into cysteamine and pantothenic acid. It is the biosynthetic precursor to the neurotransmitter hypotaurine. It is a stable aminothiol, i.e., an organic compound containing both an amine and a thiol functional groups. Cysteamine is a white, water-soluble solid. It is often used as salts of the ammonium derivative SCH2CH2NH3sup>+ including the hydrochloride, phosphocysteamine, and bitartrate. As a medication, cysteamine, sold under the brand name Cystagon among others, is indicated to treat cystinosis. Medical uses Cysteamine is used to treat cystinosis. It is available by mouth (capsule and extended release capsule) and in eye drops. When applied topically it can scavenge free radicals and lighten skin that's been darkened as a result of post-inflammatory hyperpigmentation, sun exposure and Melasma. Tent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Richard Willstatter
Richard is a male given name. It originates, via Old French, from Old Frankish and is a compound of the words descending from Proto-Germanic ''*rīk-'' 'ruler, leader, king' and ''*hardu-'' 'strong, brave, hardy', and it therefore means 'strong in rule'. Nicknames include "Richie", "Dick", "Dickon", " Dickie", "Rich", "Rick", "Rico", "Ricky", and more. Richard is a common English, German and French male name. It's also used in many more languages, particularly Germanic, such as Norwegian, Danish, Swedish, Icelandic, and Dutch, as well as other languages including Irish, Scottish, Welsh and Finnish. Richard is cognate with variants of the name in other European languages, such as the Swedish "Rickard", the Catalan "Ricard" and the Italian "Riccardo", among others (see comprehensive variant list below). People named Richard Multiple people with the same name * Richard Andersen (other) * Richard Anderson (other) * Richard Cartwright (other) * Ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back-for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |