JUNQ and IPOD on:
[Wikipedia]
[Google]
[Amazon]
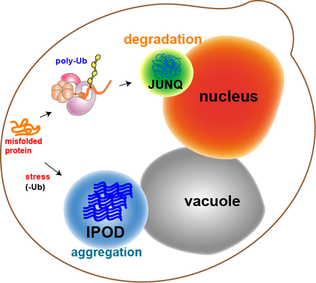 JUNQ and IPOD are types of
JUNQ and IPOD are types of
Daniel Kaganovich
showed that

Protein aggregation
Live cell imaging
Jennifer Lippincott-Schwartz : Intracellular Fluorescent Imaging: An Introduction
Susan Lindquist (MIT) : Protein Folding and Prions
Alzheimer's disease Neurodegenerative disorders Neurological disorders Structural proteins Proteins Protein complexes Protein structure Organelles Microscopy Fluorescence Cell imaging Yeasts
 JUNQ and IPOD are types of
JUNQ and IPOD are types of cytosolic
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrio ...
protein inclusion bodies
Inclusion bodies are aggregates of specific types of protein found in neurons, a number of tissue cells including red blood cells, bacteria, viruses, and plants. Inclusion bodies of aggregations of multiple proteins are also found in muscle cell ...
in eukaryotes
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bact ...
.
Neurodegenerative diseases
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic ...
, such as Parkinson's
Parkinson's disease (PD), or simply Parkinson's, is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. The symptoms usually emerge slowly, and as the disease worsens, non-motor symptoms becom ...
, Alzheimer's
Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens. It is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As t ...
, and Huntington's
Huntington's disease (HD), also known as Huntington's chorea, is a neurodegenerative disease that is mostly inherited. The earliest symptoms are often subtle problems with mood or mental abilities. A general lack of coordination and an unst ...
, are associated and correlated with protein aggregation
In molecular biology, protein aggregation is a phenomenon in which intrinsically-disordered or mis-folded proteins aggregate (i.e., accumulate and clump together) either intra- or extracellularly. Protein aggregates have been implicated in a w ...
and accumulation of misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
in inclusion bodies
Inclusion bodies are aggregates of specific types of protein found in neurons, a number of tissue cells including red blood cells, bacteria, viruses, and plants. Inclusion bodies of aggregations of multiple proteins are also found in muscle cell ...
. For many years, protein aggregation was considered a random process by which misfolded proteins stick to each other to form inclusions (imagine a bundle of hairs haphazardly piling up in a corner of a room). Moreover, protein aggregates were thought to be toxic agents and the cause for neuronal dysfunction and death. However, recent studies, using advanced methods (i.e. fluorescence microscopy
A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence microsc ...
), show that protein aggregation may actually be a tightly regulated, organized process, by which the cell protects itself from toxic proteins by sequestration to inclusion bodies. In 2008Daniel Kaganovich
showed that
eukaryotic
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bact ...
cells sort misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
into two distinct inclusion bodies in a well-managed cellular process:
# The JUNQ (JUxta Nuclear Quality control compartment)
# The IPOD (Insoluble Protein Deposit)
JUNQ and IPOD are evolutionarily conserved, and are found in specific and defined cellular sites. Delivery of misfolded, aggregated proteins to JUNQ and IPOD require an intact cytoskeleton and specific cellular quality control components, such as Heat Shock Proteins (HSPs). The partition into the two distinct inclusion bodies is due to the different handling and processing of different kinds of misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
(e.g. ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fou ...
ated vs. non-ubiquitinated proteins). Segregation of toxic protein aggregates into JUNQ and IPOD inclusion bodies is a means by which mammalian cells can be rejuvenated through asymmetric division.
Thus, the discovery of JUNQ and IPOD provided a new striking perspective of how cells manage misfolded aggregated proteins and gave convincing proof that protein aggregation
In molecular biology, protein aggregation is a phenomenon in which intrinsically-disordered or mis-folded proteins aggregate (i.e., accumulate and clump together) either intra- or extracellularly. Protein aggregates have been implicated in a w ...
is a non-random, well regulated and controlled cellular process. Furthermore, the discovery of JUNQ and IPOD suggested that in addition to temporal quality control (i.e. time dependent administration of damaged proteins) cells exploit homeostasis
In biology, homeostasis (British also homoeostasis) (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physical, and chemical conditions maintained by living systems. This is the condition of optimal functioning for the organism and i ...
spatially: If degradation isn't available, protection of the cellular environment from a misfolded protein
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
is accomplished by its sequestration to an aggregate inclusion.
Background
To function properly, mostproteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
must preserve a low-energy, three-dimensional structure known as the native state
In biochemistry, the native state of a protein or nucleic acid is its properly folded and/or assembled form, which is operative and functional. The native state of a biomolecule may possess all four levels of biomolecular structure, with the ...
. The stability of a protein is tightly regulated through all its life stages: from cradle, as it is synthesized at the ribosome
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to f ...
, through folding or assembly, till grave – when the protein is degraded and cleared from the cellular environment. Protein homeostasis
In biology, homeostasis (British also homoeostasis) (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physical, and chemical conditions maintained by living systems. This is the condition of optimal functioning for the organism and i ...
(proteostasis), results from the coordinated action of the different arms of the cellular quality control system: molecular chaperones
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assi ...
, proteases
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the form ...
and other regulatory factors. Hence, cellular viability depends on timely and efficient management of misfolded proteins. Such management, by the quality control machinery, includes recognition of the misfolded protein by chaperones and E3 ligase
A ubiquitin ligase (also called an E3 ubiquitin ligase) is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquiti ...
s, ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fou ...
ation and degradation
Degradation may refer to:
Science
* Degradation (geology), lowering of a fluvial surface by erosion
* Degradation (telecommunications), of an electronic signal
* Biodegradation of organic substances by living organisms
* Environmental degradation ...
.
Proteostasis collapse, due to damage, stress, mutations
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mitos ...
, and aging
Ageing ( BE) or aging ( AE) is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. I ...
, has been implicated as a basis for a large number of common human disorders, such as neurodegenerative diseases
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic ...
. Although caused by different kinds of mutated proteins (e.g. in Huntington's disease
Huntington's disease (HD), also known as Huntington's chorea, is a neurodegenerative disease that is mostly inherited. The earliest symptoms are often subtle problems with mood or mental abilities. A general lack of coordination and an unst ...
– the protein Huntingtin
Huntingtin (Htt) is the protein coded for in humans by the ''HTT'' gene, also known as the ''IT15'' ("interesting transcript 15") gene. Mutated ''HTT'' is the cause of Huntington's disease (HD), and has been investigated for this role and also fo ...
) and disruptive to distinct tissues (e.g. in Huntington's disease
Huntington's disease (HD), also known as Huntington's chorea, is a neurodegenerative disease that is mostly inherited. The earliest symptoms are often subtle problems with mood or mental abilities. A general lack of coordination and an unst ...
– the striatum
The striatum, or corpus striatum (also called the striate nucleus), is a nucleus (a cluster of neurons) in the subcortical basal ganglia of the forebrain. The striatum is a critical component of the motor and reward systems; receives glutamate ...
), such diseases share a common feature: accumulation of misfolded proteins in inclusion bodies
Inclusion bodies are aggregates of specific types of protein found in neurons, a number of tissue cells including red blood cells, bacteria, viruses, and plants. Inclusion bodies of aggregations of multiple proteins are also found in muscle cell ...
. Thus, it was thought that the inclusion bodies are the cause of such diseases. However, the nature and characteristics of those intra-cellular inclusion bodies stayed elusive. Different kinds of proteins (e.g. prions
Prions are misfolded proteins that have the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It i ...
, ERAD substrates) were reported to form different kinds of inclusion bodies (e.g. aggresomes In eukaryotic cells, an aggresome refers to an aggregation of misfolded proteins in the cell, formed when the protein degradation system of the cell is overwhelmed. Aggresome formation is a highly regulated process that possibly serves to organize m ...
, amyloids), yet it remained obscure if those observations combine into one and relate to the same sub-cellular site. Moreover, the pathways leading to inclusion formation and the involvement of the cellular protein quality control machinery were undefined and unknown. Thus, a systematic study providing a comprehensive understanding of protein aggregation
In molecular biology, protein aggregation is a phenomenon in which intrinsically-disordered or mis-folded proteins aggregate (i.e., accumulate and clump together) either intra- or extracellularly. Protein aggregates have been implicated in a w ...
and inclusion bodies
Inclusion bodies are aggregates of specific types of protein found in neurons, a number of tissue cells including red blood cells, bacteria, viruses, and plants. Inclusion bodies of aggregations of multiple proteins are also found in muscle cell ...
was required. The discovery of JUNQ and IPOD suggested new insights of how the cell manages different kinds of misfolded proteins and offered a novel framework for putting together the great puzzle of protein aggregation
In molecular biology, protein aggregation is a phenomenon in which intrinsically-disordered or mis-folded proteins aggregate (i.e., accumulate and clump together) either intra- or extracellularly. Protein aggregates have been implicated in a w ...
.

Discovery
The fate ofmisfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
and the process leading to the formation of aggregate inclusions, were initially studied using biochemical
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
methods (e.g. western blotting
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecti ...
).
Deeper insights into the biological process of protein quality control and aggregation was made possible by a novel approach to looking at this problem, termed "Live Cell Imaging".
Live cell imaging enables in vivo tracking of proteins in space and time, in their natural endogenous environment. Thus, such a method provides more information about the dynamics and stages of biological events and processes. The method takes advantage of the easily detectable fluorescent protein Fluorescent proteins include:
* Green fluorescent protein (GFP)
* Yellow fluorescent protein (YFP)
* Red fluorescent protein
Red fluorescent protein (RFP) is a fluorophore that fluoresces red-orange when excited. Several variants have been develo ...
s fused to a protein of interest, which can then be followed inside a cell using a fluorescence microscope
A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence micros ...
. The cell may then be treated by a perturbation of interest (e.g. a drug, expression of a misfolded protein
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
), and various properties of the fluorescently tagged protein can be assayed using time-lapse microscopy
Time-lapse microscopy is time-lapse photography applied to microscopy.
Microscope image sequences are recorded and then viewed at a greater speed to
give an accelerated view of the microscopic process.
Before the introduction of the video tape r ...
:
# Changes of the fluorescence level indicates changes of expression levels (i.e. higher levels = upregulation of a protein)
# Changes of localization (e.g. entrance of a protein from the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrio ...
to the nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucle ...
)
# Solubility (e.g. by using the FRAP assay)
# Interplay with the intracellular environment (e.g. by using the FLIP
Flip, FLIP, or flips may refer to:
People
* Flip (nickname), a list of people
* Lil' Flip (born 1981), American rapper
* Flip Simmons, Australian actor and musician
* Flip Wilson, American comedian
Arts and entertainment Fictional characters
* ...
assay)
In order to monitor the fate of cytosolic
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrio ...
misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
'' in vivo'', a plasmid
A plasmid is a small, extrachromosomal DNA molecule within a cell that is physically separated from chromosomal DNA and can replicate independently. They are most commonly found as small circular, double-stranded DNA molecules in bacteria; ho ...
carrying a GFP tagged folding reporter was cloned
Cloning is the process of producing individual organisms with identical or virtually identical DNA, either by natural or artificial means. In nature, some organisms produce clones through asexual reproduction. In the field of biotechnology, c ...
. The folding reporter, a model protein for aggregation, was a Ubc9 (SUMO-conjugating enzyme) mutant
In biology, and especially in genetics, a mutant is an organism or a new genetic character arising or resulting from an instance of mutation, which is generally an alteration of the DNA sequence of the genome or chromosome of an organism. It ...
(UBC9ts), harboring a missense mutation
In genetics, a missense mutation is a point mutation in which a single nucleotide change results in a codon that codes for a different amino acid. It is a type of nonsynonymous substitution.
Substitution of protein from DNA mutations
Missense m ...
( Y68L) with a temperature- sensitive (ts) phenotype. The marginally stable Ubc9ts is fully functional under physiological
Physiology (; ) is the scientific study of functions and mechanisms in a living system. As a sub-discipline of biology, physiology focuses on how organisms, organ systems, individual organs, cells, and biomolecules carry out the chemical ...
permissive conditions (25 °C) due to active cellular chaperones. The GFP–Ubc9ts was transformed into yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitut ...
and visualized using a fluorescence microscope
A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence micros ...
.
Monitoring the folding sensor GFP–Ubc9ts was thought to indicate the cellular proteostasis, and to assay the ability of the cellular protein quality control system to deal with various kinds of stress. It was then observed that under normal conditions, GFP–Ubc9ts is diffused in the nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucle ...
and in the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrio ...
. However, upon heat shock
The heat shock response (HSR) is a cell stress response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a norma ...
, GFP–Ubc9ts formed cytosolic punctate structures. Strikingly, when the proteasome
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases.
Proteasomes are part of a major mechanism by wh ...
was impaired and clearance of the misfolded protein by degradation
Degradation may refer to:
Science
* Degradation (geology), lowering of a fluvial surface by erosion
* Degradation (telecommunications), of an electronic signal
* Biodegradation of organic substances by living organisms
* Environmental degradation ...
was blocked, two distinct cytosolic
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrio ...
inclusions were observed to be formed. Standard and conservative biochemical methods, such as cell fractionation
In cell biology, cell fractionation is the process used to separate cellular components while preserving individual functions of each component. This is a method that was originally used to demonstrate the cellular location of various biochemical ...
and western blotting
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecti ...
would not have revealed the partition into the two types of cytosolic aggregates.
The two detected inclusions were shown to be evolutionarily conserved quality control compartments, with different characteristics and distinct functions. They were named JUNQ (JUxta Nuclear Quality control compartment) and IPOD (Insoluble Protein Deposit), and represent two cellular pathways for the sequestration and management of aggregation prone, potentially toxic proteins.
Partition of quality control substrates (i.e. misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
) to either compartment depends on their ubiquitination
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
status and aggregation state (i.e. solubility):
Proteins that are ubiquitinated are delivered to the JUNQ, where they are processed for degradation by the proteasome
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases.
Proteasomes are part of a major mechanism by wh ...
. Misfolded proteins that are not ubiquitinated and terminally aggregated are sequestered to the IPOD.
Thus, the sub-cellular location of a misfolded protein
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
(i.e. in the JUNQ or in the IPOD) provides information about its interaction with the cellular protein quality control machinery (e.g. its E3 ligase
A ubiquitin ligase (also called an E3 ubiquitin ligase) is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquiti ...
).
JUNQ
JUNQ is the JUxta Nuclear Quality control compartment.Significance
To maintain cellular homeostasis, the cellular quality control system must distinguish between folded and misfolded proteins. A misfolded protein will be recognized and tightly taken care of by either refolding or ubiquitination and proteasomal degradation. However, cellular increase of misfolded protein loads, due to various kinds of stresses (e.g.heat shock
The heat shock response (HSR) is a cell stress response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a norma ...
), may saturate and exhaust the quality control machinery. In such cases, degradation of misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
is unavailable, and a second line of active cellular defense mechanism must be executed: directing misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
to specific cellular sites.
The JUNQ serves as such a sequestration site. It was shown that when the proteasome is impaired (e.g. by low expression levels of the proteasome
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases.
Proteasomes are part of a major mechanism by wh ...
subunit RPN11), ubiquitinated
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fou ...
misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
are sorted into the JUNQ. Upon recovery from stress conditions (e.g. recovery from heat shock
The heat shock response (HSR) is a cell stress response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a norma ...
at a permissive temperature), misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
that accumulate in the JUNQ may be either refolded by the cellular chaperone machinery, or degraded by the 26S proteasome
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases.
Proteasomes are part of a major mechanism by whi ...
. Thus, the sequestration of a protein to the JUNQ is reversible.
Properties
The JUNQ is a non- membrane bound cellular site located in a margin of the nucleus, in close proximity to theendoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ( ...
. FRAP and FLIP
Flip, FLIP, or flips may refer to:
People
* Flip (nickname), a list of people
* Lil' Flip (born 1981), American rapper
* Flip Simmons, Australian actor and musician
* Flip Wilson, American comedian
Arts and entertainment Fictional characters
* ...
assays revealed that proteins in the JUNQ are soluble and exchange with the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrio ...
, suggesting that the JUNQ has a dynamic structure.
Delivery to the JUNQ depends on molecular chaperones
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assi ...
and co-chaperone
Co-chaperones are proteins that assist chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly essential in stimulatio ...
s and on the actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ov ...
cytoskeleton. Misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
must be ubiquitinated
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fou ...
to be sorted to the JUNQ. If ubiquitination
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
is blocked, a misfolded protein
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
will be directed to the IPOD inclusion. Misfolded protein
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
accumulation recruits 26S proteasomes to the JUNQ.
IPOD
IPOD is the Insoluble Protein Deposit compartment.Significance
It is becoming more evident that the cellular capacity to maintainproteostasis Proteostasis is the dynamic regulation of a balanced, functional proteome. The proteostasis network includes competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking, and degradation of protei ...
declines with age, thereby causing the late onset of neurodegenerative
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic ...
diseases. In such diseases (e.g. Huntington's disease
Huntington's disease (HD), also known as Huntington's chorea, is a neurodegenerative disease that is mostly inherited. The earliest symptoms are often subtle problems with mood or mental abilities. A general lack of coordination and an unst ...
), a mutated
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mitosi ...
protein misfolds and becomes toxic to the cellular environment by various ways such as denaturating cytosolic proteins. Incompetent of degrading those toxic species, the cell must isolate them to avoid their hazardous interaction with the cellular proteome
The proteome is the entire set of proteins that is, or can be, expressed by a genome, cell, tissue, or organism at a certain time. It is the set of expressed proteins in a given type of cell or organism, at a given time, under defined conditions ...
. The IPOD was shown to be the sub-cellular site to which toxic amyloidogenic proteins are sequestered to, hereby serving as a protective quality control compartment.
In addition, it was suggested by the Lindquist group, that the IPOD is the site where yeast prions undergo a maturation process. Thus, the IPOD may serve not only as a sequestration site, but also as a functional compartment.
Properties
The IPOD is a non- membrane bound cellular site, which in yeast is located by thevacuole
A vacuole () is a membrane-bound organelle which is present in plant and fungal cells and some protist, animal, and bacterial cells. Vacuoles are essentially enclosed compartments which are filled with water containing inorganic and organic mo ...
. FRAP and FLIP
Flip, FLIP, or flips may refer to:
People
* Flip (nickname), a list of people
* Lil' Flip (born 1981), American rapper
* Flip Simmons, Australian actor and musician
* Flip Wilson, American comedian
Arts and entertainment Fictional characters
* ...
assays revealed that proteins in the IPOD are tightly packed, in-soluble and don't exchange with the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrio ...
. Amyloidogenic proteins, such as the Huntingtin
Huntingtin (Htt) is the protein coded for in humans by the ''HTT'' gene, also known as the ''IT15'' ("interesting transcript 15") gene. Mutated ''HTT'' is the cause of Huntington's disease (HD), and has been investigated for this role and also fo ...
protein, are the IPOD's substrates.
Misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
must be non-ubiquitinated
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fou ...
to be sorted to the IPOD. Ubiquitination of an otherwise IPOD substrate, such as the RNQ1 fungal prion, will result in its sequestration in the JUNQ inclusion.
Upon accumulation of misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
, the disaggregase chaperone, AAA protein
AAA, Triple A, or Triple-A is a three-letter initialism or abbreviation which may refer to:
Airports
* Anaa Airport in French Polynesia (IATA airport code AAA)
* Logan County Airport (Illinois) (FAA airport code AAA)
Arts, entertainment, and me ...
HSP104, localizes to the IPOD. It is yet to be determined if HSP104 functions in the IPOD or is simply sequestered there being hooked to a substrate.
The pre-autophagosomal structure (PAS) is localized by the IPOD. However, it wasn't shown that IPOD substrates are delivered to the vacuole, and so the link between the IPOD and autophagy
Autophagy (or autophagocytosis; from the Ancient Greek , , meaning "self-devouring" and , , meaning "hollow") is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent r ...
is yet to be determined.
See also
*Confocal laser scanning microscopy
Confocal microscopy, most frequently confocal laser scanning microscopy (CLSM) or laser confocal scanning microscopy (LCSM), is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of using a sp ...
* Protein aggregation
In molecular biology, protein aggregation is a phenomenon in which intrinsically-disordered or mis-folded proteins aggregate (i.e., accumulate and clump together) either intra- or extracellularly. Protein aggregates have been implicated in a w ...
References
{{ReflistExternal links
Protein aggregation
Live cell imaging
Jennifer Lippincott-Schwartz : Intracellular Fluorescent Imaging: An Introduction
Susan Lindquist (MIT) : Protein Folding and Prions
Alzheimer's disease Neurodegenerative disorders Neurological disorders Structural proteins Proteins Protein complexes Protein structure Organelles Microscopy Fluorescence Cell imaging Yeasts