International Avogadro Project on:
[Wikipedia]
[Google]
[Amazon]
The scientific community examined several approaches to redefining the  The International Committee for Weights and Measures (CIPM) approved a redefinition of the SI base units in November 2018 that defines the kilogram by defining the Planck constant to be exactly . This approach effectively defines the kilogram in terms of the second and the metre, and took effect on 20 May 2019.
In 1960, the metre, previously similarly having been defined with reference to a single platinum-iridium bar with two marks on it, was redefined in terms of an invariant physical constant (the wavelength of a particular emission of light emitted by krypton, and later the speed of light) so that the standard can be independently reproduced in different laboratories by following a written specification.
At the 94th Meeting of the International Committee for Weights and Measures (CIPM) in 2005, it was recommended that the same be done with the kilogram.
In October 2010, the CIPM voted to submit a resolution for consideration at the
The International Committee for Weights and Measures (CIPM) approved a redefinition of the SI base units in November 2018 that defines the kilogram by defining the Planck constant to be exactly . This approach effectively defines the kilogram in terms of the second and the metre, and took effect on 20 May 2019.
In 1960, the metre, previously similarly having been defined with reference to a single platinum-iridium bar with two marks on it, was redefined in terms of an invariant physical constant (the wavelength of a particular emission of light emitted by krypton, and later the speed of light) so that the standard can be independently reproduced in different laboratories by following a written specification.
At the 94th Meeting of the International Committee for Weights and Measures (CIPM) in 2005, it was recommended that the same be done with the kilogram.
In October 2010, the CIPM voted to submit a resolution for consideration at the
 The
The
 Another Avogadro constant-based approach, known as the International Avogadro Coordination's ''Avogadro project'', would define and delineate the kilogram as a 93.6mm diameter sphere of silicon atoms. Silicon was chosen because a commercial infrastructure with
Another Avogadro constant-based approach, known as the International Avogadro Coordination's ''Avogadro project'', would define and delineate the kilogram as a 93.6mm diameter sphere of silicon atoms. Silicon was chosen because a commercial infrastructure with
Working group 1.24, Ion Accumulation
/ref> Follow-on experiments using bismuth ions and a current of 30mA were expected to accumulate a mass of 30g in six days and to have a relative uncertainty of better than 1 ppm. Ultimately, ionaccumulation approaches proved to be unsuitable. Measurements required months and the data proved too erratic for the technique to be considered a viable future replacement to the IPK. Among the many technical challenges of the ion-deposition apparatus was obtaining a sufficiently high ion current (mass deposition rate) while simultaneously decelerating the ions so they could all deposit onto a target electrode embedded in a balance pan. Experiments with gold showed the ions had to be decelerated to very low energies to avoid sputtering effects—a phenomenon whereby ions that had already been counted ricochet off the target electrode or even dislodged atoms that had already been deposited. The deposited mass fraction in the 2003 German experiments only approached very close to 100% at ion energies of less than around (<1km/s for gold). If the kilogram had been defined as a precise quantity of gold or bismuth atoms deposited with an electric current, not only would the Avogadro constant and the atomic mass of gold or bismuth have to have been precisely fixed, but also the value of the
 This approach would define the kilogram as "the mass which would be accelerated at precisely when subjected to the per-metre force between two straight parallel conductors of infinite length, of negligible circular cross section, placed one metre apart in vacuum, through which flow a constant current of elementary charges per second".
Effectively, this would define the kilogram as a derivative of the
This approach would define the kilogram as "the mass which would be accelerated at precisely when subjected to the per-metre force between two straight parallel conductors of infinite length, of negligible circular cross section, placed one metre apart in vacuum, through which flow a constant current of elementary charges per second".
Effectively, this would define the kilogram as a derivative of the
kilogram
The kilogram (also kilogramme) is the unit of mass in the International System of Units (SI), having the unit symbol kg. It is a widely used measure in science, engineering and commerce worldwide, and is often simply called a kilo colloquially ...
before deciding on a redefinition of the SI base units
In 2019, four of the seven SI base units specified in the International System of Quantities were redefined in terms of natural physical constants, rather than human artifacts such as the standard kilogram.
Effective 20 May 2019, the 144th ...
in November 2018. Each approach had advantages and disadvantages.
Prior to the redefinition the kilogram, and several other SI units based on the kilogram, were defined by an artificial metal object called the international prototype of the kilogram
The International Prototype of the Kilogram (referred to by metrologists as the IPK or Le Grand K; sometimes called the '' ur-kilogram,'' or ''urkilogram,'' particularly by German-language authors writing in English) is an object that was used t ...
(IPK). There was broad agreement that the older definition of the kilogram should be replaced.
General Conference on Weights and Measures
The General Conference on Weights and Measures (GCWM; french: Conférence générale des poids et mesures, CGPM) is the supreme authority of the International Bureau of Weights and Measures (BIPM), the intergovernmental organization established i ...
(CGPM), to "take note of an intention" that the kilogram be defined in terms of the Planck constant, (which has dimensions of energy times time) together with other physical constants. This resolution was accepted by the 24th conference of the CGPM in October 2011 and further discussed at the 25th conference in 2014. Although the Committee recognised that significant progress had been made, they concluded that the data did not yet appear sufficiently robust to adopt the revised definition, and that work should continue to enable the adoption at the 26th meeting, scheduled for 2018. Such a definition would theoretically permit any apparatus that was capable of delineating the kilogram in terms of the Planck constant to be used as long as it possessed sufficient precision, accuracy and stability. The Kibble balance
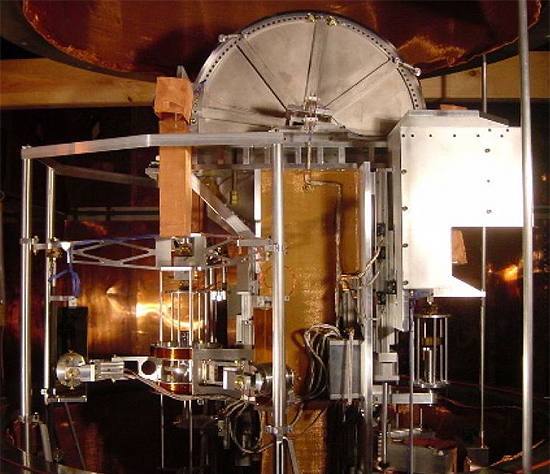
A Kibble balance is an electromechanical measuring instrument that measures the weight of a test object very precisely by the electric current and voltage needed to produce a compensating force. It is a metrological instrument that can realize t ...
is one way do this.
As part of this project, a variety of very different technologies and approaches were considered and explored over many years. Some of these approaches were based on equipment and procedures that would have enabled the reproducible production of new, kilogram-mass prototypes on demand using measurement techniques and material properties that are ultimately based on, or traceable to, physical constants. Others were based on devices that measured either the acceleration or weight of hand-tuned kilogram test masses and which expressed their magnitudes in electrical terms via special components that permit traceability to physical constants. Such approaches depend on converting a weight measurement to a mass, and therefore require the precise measurement of the strength of gravity in laboratories. All approaches would have precisely fixed one or more constants of nature at a defined value.
Kibble balance
 The
The Kibble balance
A Kibble balance is an electromechanical measuring instrument that measures the weight of a test object very precisely by the electric current and voltage needed to produce a compensating force. It is a metrological instrument that can realize t ...
(known as a "watt balance" before 2016) is essentially a single-pan weighing scale that measures the electric power
Electric power is the rate at which electrical energy is transferred by an electric circuit. The SI unit of power is the watt, one joule per second. Standard prefixes apply to watts as with other SI units: thousands, millions and billions o ...
necessary to oppose the weight of a kilogram test mass as it is pulled by Earth's gravity. It is a variation of an ampere balance
The ampere balance (also current balance or Kelvin balance) is an electromechanical apparatus used for the precise measurement of the SI unit of electric current, the ampere. It was invented by William Thomson, 1st Baron Kelvin.
The curre ...
, with an extra calibration step that eliminates the effect of geometry. The electric potential in the Kibble balance is delineated by a Josephson voltage standard
A Josephson voltage standard is a complex system that uses a superconducting integrated circuit chip operating at a temperature of 4 K to generate stable voltages that depend only on an applied frequency and fundamental constants. It is an int ...
, which allows voltage to be linked to an invariant constant of nature with extremely high precision and stability. Its circuit resistance
Resistance may refer to:
Arts, entertainment, and media Comics
* Either of two similarly named but otherwise unrelated comic book series, both published by Wildstorm:
** ''Resistance'' (comics), based on the video game of the same title
** ''T ...
is calibrated against a quantum Hall effect resistance standard.
The Kibble balance requires extremely precise measurement of the local gravitational acceleration ''g'' in the laboratory, using a gravimeter. For instance when the elevation of the centre of the gravimeter differs from that of the nearby test mass in the Kibble balance, the NIST compensates for Earth's gravity gradient of 309 μGal per metre, which affects the weight of a one-kilogram test mass by about 316μg/m.
In April 2007, the NIST's implementation of the Kibble balance demonstrated a combined relative standard uncertainty (CRSU) of 36μg.The combined relative standard uncertainty (CRSU) of these measurements, as with all other tolerances and uncertainties in this article unless otherwise noted, are at one standard deviation (1''σ''), which equates to a confidence level of about 68%; that is to say, 68% of the measurements fall within the stated tolerance. The UK's National Physical Laboratory's Kibble balance demonstrated a CRSU of 70.3μg in 2007. That Kibble balance was disassembled and shipped in 2009 to Canada's Institute for National Measurement Standards (part of the National Research Council), where research and development with the device could continue.
Gravity and the nature of the Kibble balance, which oscillates test masses up and down against the local gravitational acceleration ''g'', are exploited so that mechanical power is compared against electrical power, which is the square of voltage divided by electrical resistance. However, ''g'' varies significantly—by nearly 1%—depending on where on the Earth's surface the measurement is made (see '' Earth's gravity''). There are also slight seasonal variations in ''g'' at a location due to changes in underground water tables, and larger semimonthly and diurnal changes due to tidal distortions in the Earth's shape caused by the Moon and the Sun. Although ''g'' would not be a term in the ''definition'' of the kilogram, it would be crucial in the process of measurement of the kilogram when relating energy to power. Accordingly, ''g'' must be measured with at least as much precision and accuracy as are the other terms, so measurements of ''g'' must also be traceable to fundamental constants of nature. For the most precise work in mass metrology, ''g'' is measured using dropping-mass absolute gravimeters that contain an iodine-stabilised helium–neon laser interferometer
Interferometry is a technique which uses the ''interference'' of superimposed waves to extract information. Interferometry typically uses electromagnetic waves and is an important investigative technique in the fields of astronomy, fiber op ...
. The fringe-signal, frequency-sweep output from the interferometer is measured with a rubidium atomic clock. Since this type of dropping-mass gravimeter derives its accuracy and stability from the constancy of the speed of light as well as the innate properties of helium, neon, and rubidium atoms, the 'gravity' term in the delineation of an all-electronic kilogram is also measured in terms of invariants of nature—and with very high precision. For instance, in the basement of the NIST's Gaithersburg facility in 2009, when measuring the gravity acting upon Pt10Ir test masses (which are denser, smaller, and have a slightly lower center of gravity inside the Kibble balance than stainless steel masses), the measured value was typically within 8 ppb of .
The virtue of electronic realisations like the Kibble balance is that the definition and dissemination of the kilogram no longer depends upon the stability of kilogram prototypes, which must be very carefully handled and stored. It frees physicists from the need to rely on assumptions about the stability of those prototypes. Instead, hand-tuned, close-approximation mass standards can simply be weighed and documented as being equal to one kilogram plus an offset value. With the Kibble balance, while the kilogram is ''delineated'' in electrical and gravity terms, all of which are traceable to invariants of nature; it is ''defined'' in a manner that is directly traceable to three fundamental constants of nature. The Planck constant defines the kilogram in terms of the second and the metre. By fixing the Planck constant, the ''definition'' of the kilogram depends in addition only on the ''definitions'' of the second and the metre. The definition of the second depends on a single defined physical constant: the ground state hyperfine splitting frequency of the caesium-133 atom . The metre depends on the second and on an additional defined physical constant: the speed of light . With the kilogram redefined in this manner, physical objects such as the IPK are no longer part of the definition, but instead become ''transfer standards''.
Scales like the Kibble balance also permit more flexibility in choosing materials with especially desirable properties for mass standards. For instance, Pt10Ir could continue to be used so that the specific gravity of newly produced mass standards would be the same as existing national primary and check standards (≈21.55g/ml). This would reduce the relative uncertainty when making mass comparisons in air. Alternatively, entirely different materials and constructions could be explored with the objective of producing mass standards with greater stability. For instance, osmium
Osmium (from Greek grc, ὀσμή, osme, smell, label=none) is a chemical element with the symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mos ...
-iridium alloys could be investigated if platinum's propensity to absorb hydrogen (due to catalysis of VOCs and hydrocarbon-based cleaning solvents) and atmospheric mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
proved to be sources of instability. Also, vapor-deposited, protective ceramic coatings like nitrides could be investigated for their suitability for chemically isolating these new alloys.
The challenge with Kibble balances is not only in reducing their uncertainty, but also in making them truly ''practical'' realisations of the kilogram. Nearly every aspect of Kibble balances and their support equipment requires such extraordinarily precise and accurate, state-of-the-art technology that—unlike a device like an atomic clock—few countries would currently choose to fund their operation. For instance, the NIST's Kibble balance used four resistance standards in 2007, each of which was rotated through the Kibble balance every two to six weeks after being calibrated in a different part of NIST headquarters facility in Gaithersburg, Maryland. It was found that simply moving the resistance standards down the hall to the Kibble balance after calibration altered their values 10 ppb (equivalent to 10μg) or more. Present-day technology is insufficient to permit stable operation of Kibble balances between even biannual calibrations. When the new definition takes effect, it is likely there will only be a few—at most—Kibble balances initially operating in the world.
Alternative approaches to redefining the kilogram
Several alternative approaches to redefining the kilogram that were fundamentally different from the Kibble balance were explored to varying degrees, with some abandoned. The Avogadro project, in particular, was important for the 2018 redefinition decision because it provided an accurate measurement of the Planck constant that was consistent with and independent of the Kibble balance method. The alternative approaches included:Atom-counting approaches
Avogadro project
mature technology
A mature technology is a technology that has been in use for long enough that most of its initial faults and inherent problems have been removed or reduced by further development. In some contexts, it may also refer to technology that has not se ...
for creating defect-free, ultra-pure monocrystalline silicon already exists, the Czochralski process, to service the semiconductor industry.
To make a practical realisation of the kilogram, a silicon boule (a rod-like, single-crystal ingot) would be produced. Its isotopic composition would be measured with a mass spectrometer to determine its average relative atomic mass. The boule would be cut, ground, and polished into spheres. The size of a select sphere would be measured using optical interferometry
Interferometry is a technique which uses the ''interference'' of superimposed waves to extract information. Interferometry typically uses electromagnetic waves and is an important investigative technique in the fields of astronomy, fiber opt ...
to an uncertainty of about 0.3nm on the radius—roughly a single atomic layer. The precise lattice spacing between the atoms in its crystal structure (≈192pm) would be measured using a scanning X-ray interferometer An X-ray interferometer is analogous to a neutron interferometer. It has been suggested that it may offer the very highest spatial resolution in astronomy, though the technology is unproven as of 2008.
One technique is triple Laue interferometry ...
. This permits its atomic spacing to be determined with an uncertainty of only three parts per billion. With the size of the sphere, its average atomic mass, and its atomic spacing known, the required sphere diameter can be calculated with sufficient precision and low uncertainty to enable it to be finish-polished to a target mass of one kilogram.
Experiments are being performed on the Avogadro Project's silicon spheres to determine whether their masses are most stable when stored in a vacuum, a partial vacuum, or ambient pressure. However, no technical means currently exist to prove a long-term stability any better than that of the IPK's, because the most sensitive and accurate measurements of mass are made with dual-pan balances like the BIPM's FB2 flexure-strip balance (see ', below). Balances can only compare the mass of a silicon sphere to that of a reference mass. Given the latest understanding of the lack of long-term mass stability with the IPK and its replicas, there is no known, perfectly stable mass artefact to compare against. Single-pan scale
Scale or scales may refer to:
Mathematics
* Scale (descriptive set theory), an object defined on a set of points
* Scale (ratio), the ratio of a linear dimension of a model to the corresponding dimension of the original
* Scale factor, a number ...
s, which measure weight relative to an invariant of nature, are not precise to the necessary long-term uncertainty of 10–20 parts per billion. Another issue to be overcome is that silicon oxidises and forms a thin layer (equivalent to silicon atoms deep) of silicon dioxide ( quartz) and silicon monoxide. This layer slightly increases the mass of the sphere, an effect that must be accounted for when polishing the sphere to its finished size. Oxidation is not an issue with platinum and iridium, both of which are noble metals that are roughly as cathodic as oxygen and therefore don't oxidise unless coaxed to do so in the laboratory. The presence of the thin oxide layer on a silicon-sphere mass prototype places additional restrictions on the procedures that might be suitable to clean it to avoid changing the layer's thickness or oxide stoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equal ...
.
All silicon-based approaches would fix the Avogadro constant but vary in the details of the definition of the kilogram. One approach would use silicon with all three of its natural isotopes present. About 7.78% of silicon comprises the two heavier isotopes: 29Si and 30Si. As described in ' below, this method would ''define'' the magnitude of the kilogram in terms of a certain number of 12C atoms by fixing the Avogadro constant; the silicon sphere would be the ''practical realisation''. This approach could accurately delineate the magnitude of the kilogram because the masses of the three silicon nuclide
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by Truman ...
s relative to 12C are known with great precision (relative uncertainties of 1 ppb or better). An alternative method for creating a silicon sphere-based kilogram proposes to use isotopic separation techniques to enrich the silicon until it is nearly pure 28Si, which has a relative atomic mass of . With this approach, the Avogadro constant would not only be fixed, but so too would the atomic mass of 28Si. As such, the definition of the kilogram would be decoupled from 12C and the kilogram would instead be defined as atoms of 28Si (≈ fixed moles of 28Si atoms). Physicists could elect to define the kilogram in terms of 28Si even when kilogram prototypes are made of natural silicon (all three isotopes present). Even with a kilogram definition based on theoretically pure 28Si, a silicon-sphere prototype made of only nearly pure 28Si would necessarily deviate slightly from the defined number of moles of silicon to compensate for various chemical and isotopic impurities as well as the effect of surface oxides.
Carbon-12
Though not offering a practical realisation, this definition would precisely define the magnitude of the kilogram in terms of a certain number ofcarbon12
Carbon12 is a wooden building in Portland, Oregon's Eliot neighborhood, in the United States. The eight-story structure built with Oregon-made cross-laminated timber (CLT) became the tallest wood building in the United States upon its completion.< ...
atoms. Carbon12 (12C) is an isotope of carbon. The mole is currently defined as "the quantity of entities (elementary particles like atoms or molecules) equal to the number of atoms in 12 grams of carbon12". Thus, the current definition of the mole requires that moles ( mol) of 12C has a mass of precisely one kilogram. The number of atoms in a mole, a quantity known as the Avogadro constant, is experimentally determined, and the current best estimate of its value is This new definition of the kilogram proposed to fix the Avogadro constant at precisely with the kilogram being defined as "the mass equal to that of atoms of 12C".
The accuracy of the measured value of the Avogadro constant is currently limited by the uncertainty in the value of the Planck constant. That relative standard uncertainty has been 50parts per billion (ppb) since 2006. By fixing the Avogadro constant, the practical effect of this proposal would be that the uncertainty in the mass of a 12C atom—and the magnitude of the kilogram—could be no better than the current 50ppb uncertainty in the Planck constant. Under this proposal, the magnitude of the kilogram would be subject to future refinement as improved measurements of the value of the Planck constant become available; electronic realisations of the kilogram would be recalibrated as required. Conversely, an electronic ''definition'' of the kilogram (see ', below), which would precisely fix the Planck constant, would continue to allow moles of 12C to have a mass of precisely one kilogram but the number of atoms comprising a mole (the Avogadro constant) would continue to be subject to future refinement.
A variation on a 12C-based definition proposes to define the Avogadro constant as being precisely 3 (≈) atoms. An imaginary realisation of a 12-gram mass prototype would be a cube of 12C atoms measuring precisely atoms across on a side. With this proposal, the kilogram would be defined as "the mass equal to 3× atoms of 12C."
Ion accumulation
Another Avogadro-based approach, ion accumulation, since abandoned, would have defined and delineated the kilogram by precisely creating new metal prototypes on demand. It would have done so by accumulating gold or bismuth ions (atoms stripped of an electron) and counting them by measuring the electric current required to neutralise the ions. Gold (197Au) and bismuth (209Bi) were chosen because they can be safely handled and have the two highest atomic masses among themononuclidic elements
A mononuclidic element or monotopic element is one of the 21 chemical elements that is found naturally on Earth essentially as a single nuclide (which may, or may not, be a stable nuclide). This single nuclide will have a characteristic atomic m ...
that are stable (gold) or effectively so (bismuth).In 2003, the same year the first gold-deposition experiments were conducted, physicists found that the only naturally occurring isotope of bismuth, 209Bi, is actually very slightly radioactive, with the longest known radioactive half-life of any naturally occurring element that decays via alpha radiation—a half-life of . As this is 1.4 billion times the age of the universe, 209Bi is considered a stable isotope for most practical applications (those unrelated to such disciplines as nucleocosmochronology and geochronology). In other terms, of the bismuth that existed on Earth 4.567 billion years ago still exists today. Only two mononuclidic elements are heavier than bismuth and only one approaches its stability: thorium. Long considered a possible replacement for uranium in nuclear reactors, thorium can cause cancer when inhaled because it is over 1.2billion times more radioactive than bismuth. It also has such a strong tendency to oxidise that its powders are pyrophoric. These characteristics make thorium unsuitable in ion-deposition experiments. See also ''Isotopes of bismuth
Bismuth (83Bi) has 41 known isotopes, ranging from 184Bi to 224Bi. Bismuth has no stable isotopes, but does have one very long-lived isotope; thus, the standard atomic weight can be given as . Although bismuth-209 is now known to be unstable, it h ...
'', ''Isotopes of gold
Gold (79Au) has one stable isotope, 197Au, and 36 radioisotopes, with 195Au being the most stable with a half-life of 186 days. Gold is currently considered the heaviest monoisotopic element. Bismuth formerly held that distinction until alpha-deca ...
'' and '' Isotopes of thorium''. See also '' Table of nuclides''.
With a gold-based definition of the kilogram for instance, the relative atomic mass of gold could have been fixed as precisely , from the current value of . As with a definition based upon carbon12, the Avogadro constant would also have been fixed. The kilogram would then have been defined as "the mass equal to that of precisely atoms of gold" (precisely 3,057,443,620,887,933,963,384,315 atoms of gold or about fixed moles).
In 2003, German experiments with gold at a current of only demonstrated a relative uncertainty of 1.5%.The German national metrology institute, known as the Physikalisch-Technische Bundesanstalt (PTB)Working group 1.24, Ion Accumulation
/ref> Follow-on experiments using bismuth ions and a current of 30mA were expected to accumulate a mass of 30g in six days and to have a relative uncertainty of better than 1 ppm. Ultimately, ionaccumulation approaches proved to be unsuitable. Measurements required months and the data proved too erratic for the technique to be considered a viable future replacement to the IPK. Among the many technical challenges of the ion-deposition apparatus was obtaining a sufficiently high ion current (mass deposition rate) while simultaneously decelerating the ions so they could all deposit onto a target electrode embedded in a balance pan. Experiments with gold showed the ions had to be decelerated to very low energies to avoid sputtering effects—a phenomenon whereby ions that had already been counted ricochet off the target electrode or even dislodged atoms that had already been deposited. The deposited mass fraction in the 2003 German experiments only approached very close to 100% at ion energies of less than around (<1km/s for gold). If the kilogram had been defined as a precise quantity of gold or bismuth atoms deposited with an electric current, not only would the Avogadro constant and the atomic mass of gold or bismuth have to have been precisely fixed, but also the value of the
elementary charge
The elementary charge, usually denoted by is the electric charge carried by a single proton or, equivalently, the magnitude of the negative electric charge carried by a single electron, which has charge −1 . This elementary charge is a fundame ...
(''e''), likely to (from the currently recommended value of ). Doing so would have effectively defined the ampere
The ampere (, ; symbol: A), often shortened to amp,SI supports only the use of symbols and deprecates the use of abbreviations for units. is the unit of electric current in the International System of Units (SI). One ampere is equal to elect ...
as a flow of electrons per second past a fixed point in an electric circuit. The SI unit of mass would have been fully defined by having precisely fixed the values of the Avogadro constant and elementary charge, and by exploiting the fact that the atomic masses of bismuth and gold atoms are invariant, universal constants of nature.
Beyond the slowness of making a new mass standard and the poor reproducibility, there were other intrinsic shortcomings to the ionaccumulation approach that proved to be formidable obstacles to ion-accumulation-based techniques becoming a practical realisation. The apparatus necessarily required that the deposition chamber have an integral balance system to enable the convenient calibration of a reasonable quantity of transfer standards relative to any single internal ion-deposited prototype. Furthermore, the mass prototypes produced by ion deposition techniques would have been nothing like the freestanding platinum-iridium prototypes currently in use; they would have been deposited onto—and become part of—an electrode imbedded into one pan of a special balance integrated into the device. Moreover, the ion-deposited mass wouldn't have had a hard, highly polished surface that can be vigorously cleaned like those of current prototypes. Gold, while dense and a noble metal (resistant to oxidation and the formation of other compounds), is extremely soft so an internal gold prototype would have to be kept well isolated and scrupulously clean to avoid contamination and the potential of wear from having to remove the contamination. Bismuth, which is an inexpensive metal used in low-temperature solders, slowly oxidises when exposed to room-temperature air and forms other chemical compounds and so would not have produced stable reference masses unless it was continually maintained in a vacuum or inert atmosphere.
Ampere-based force
 This approach would define the kilogram as "the mass which would be accelerated at precisely when subjected to the per-metre force between two straight parallel conductors of infinite length, of negligible circular cross section, placed one metre apart in vacuum, through which flow a constant current of elementary charges per second".
Effectively, this would define the kilogram as a derivative of the
This approach would define the kilogram as "the mass which would be accelerated at precisely when subjected to the per-metre force between two straight parallel conductors of infinite length, of negligible circular cross section, placed one metre apart in vacuum, through which flow a constant current of elementary charges per second".
Effectively, this would define the kilogram as a derivative of the ampere
The ampere (, ; symbol: A), often shortened to amp,SI supports only the use of symbols and deprecates the use of abbreviations for units. is the unit of electric current in the International System of Units (SI). One ampere is equal to elect ...
rather than the present relationship, which defines the ampere as a derivative of the kilogram. This redefinition of the kilogram would specify elementary charge
The elementary charge, usually denoted by is the electric charge carried by a single proton or, equivalently, the magnitude of the negative electric charge carried by a single electron, which has charge −1 . This elementary charge is a fundame ...
(''e'') as precisely coulomb
The coulomb (symbol: C) is the unit of electric charge in the International System of Units (SI).
In the present version of the SI it is equal to the electric charge delivered by a 1 ampere constant current in 1 second and to elementary char ...
rather than the current recommended value of It would necessarily follow that the ampere (one coulomb per second) would also become an electric current of this precise quantity of elementary charges per second passing a given point in an electric circuit.
The virtue of a practical realisation based upon this definition is that unlike the Kibble balance and other scale-based methods, all of which require the careful characterisation of gravity in the laboratory, this method delineates the magnitude of the kilogram directly in the very terms that define the nature of mass: acceleration due to an applied force. Unfortunately, it is extremely difficult to develop a practical realisation based upon accelerating masses. Experiments over a period of years in Japan with a superconducting, 30g mass supported by diamagnetic levitation never achieved an uncertainty better than ten parts per million. Magnetic hysteresis was one of the limiting issues. Other groups performed similar research that used different techniques to levitate the mass.
Notes
References
{{reflist SI base units Units of mass